-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Life Sciences
p-ISSN: 2163-1387 e-ISSN: 2163-1395
2012; 2(3): 52-56
doi: 10.5923/j.als.20120203.02
Sub-cytotoxic Concentration of AflatoxinB2 Prevents NO-Mediated Increased Mitochondrial Membrane Potential and Intracellular Killing of Candida albicans in MacrophagesRunning Title: Changes of Nitric Oxide and Mitochondrial Membrane Potential by Aflatoxin
Dilip Chatterjee1, Pratiti Ghosh2
1Centre for Agri-Business Science & Knowledge Transfer, Department of Food & Nutrition, West Bengal State University Kolkata, India
2Department of Physiology, West Bengal State University, Kolkata, India
Correspondence to: Pratiti Ghosh, Department of Physiology, West Bengal State University, Kolkata, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Aflatoxin B2 (AFB2) is a carcinogenic and immunotoxic metabolite produced by Aspergillus flavus growing on crops and food. The immune functions of rat macrophages when exposed to sub-cytotoxic concentrations of AFB2 which may make exposed people susceptible to Candida albicans infections, have been assessed. This level of AFB2 significantly prevents immune functions of macrophages by inhibiting nitric oxide (NO) production, mitochondrial membrane potential and intracellular killing of C.albicans by macrophages even in the presence of activating signal, interferon-γ. AFB2-induced prevention of increased membrane potential and intracellular killing of C.albicans by macrophages may be due to the prevention of iNOS (inducible nitric oxide synthetase) activity which results in the suppression of NO production. This study provides an important clue to the possibility that macrophages may give refuge to C.albicans for survival and dissemination and thus C.albicans is capable of inducing self-protective effect by the action of AFB2. The presence of AFB2 would thus help the macrophages to allow the C.albicans to replicate and persist, while being invisible to the immune system. This plausibility will give impetus to medicinal development.
Keywords: Aflatoxin B2, Candida Albicans, Nitric Oxide, Membrane Potential, Macrophage, Interferon-γ, Phagocytosis
Article Outline
1. Introduction
1.1. Aflatoxin
- Aflatoxins are mycotoxins formed as metabolites by certain Aspergillus species (A. Flavus, A. parasiticus, A. nomius and A. niger) in/on foods and feeds. Aflatoxicosis affects agriculture, food, animals subsisting on them and also exposed humans to the extent of mortality. Further, aflatoxin impairs resistance to mycosis induced by Candida albicans and this immunoresistance to Candida infliction decreases with the dosage of aflatoxin. Of the four major aflatoxins (B1, B2, G1 & G2), infection by the aflatoxin B2(AFB2) variety has been characterized by reduction in overall growth, liverThe mechanism of action of aflatoxinB2 is well established but the immunosuppressive aspect of sub-cytotoxic concentration of AFB2 has not yet been detailed. So, this paper looks into the effects of AFB2 on phagocytosis and intracellular killing of Candida albicans by rat peritoneal macrophages. The response of macrophages to AFB2 may be implicated in the effects of AFB2 on nitric oxide (NO) production[7]. The present authors are interested in the effects of sub-cytotoxic concentrations of AFB2 on the immune functions of macrophages which make AFB2-exposed people susceptible to fungal infections.
1.2. Macrophage and Phagocytosis
- Macrophages from different mammalian species have the ability to phagocytose C. albicans but only the yeast forms are killed intracellularly. Moreover, several macrophage-secretory products including nitric oxide are generated in antimicrobial response. Several publications have shown the role of nitric oxide on host-C. albicans interaction. Therefore it will be important to go some steps further to study the mechanism(s) involved in the inhibitory effect of AFB2 on macrophage functions[8]. Cusumano et al in 1990, showed the effect of AFB2 on phagocytosis and intracellular killing on rat peritoneal macrophages. In addition, the production of nitric oxide has been implicated in macrophage mediated phagocytosis and intracellular killing[10].
1.3. Membrane Potential
- Both nitric oxide and tumor necrosis factor alfa (TNF-α), produced in stimulated macrophages can promote the uncoupling of electron transport from ATP production, which results in decrease of mitochondrial membrane potential (ѱm) and opening of mitochondrial permeability transition (PT) pore through which cytochrome c is released from mitochondria into the cytosol of macrophage cells[11, 12]. Thereafter a cascade of events trigger macrophage mediated cytotoxicity and intracellular killing[13]. Immunological stimulus such as interferon-γ, transmits signals to macrophage nucleus, activating the latter to express cytokine inducible nitric oxide synthase (iNOS), which catalyzes the synthesis of high concentration of NO from L-arginine and molecular oxygen. NO thus produced, kills tumor cells, bacteria and fungi[14].The findings outlined above, prompted us to study the involvement of mitochondria related events in nitric oxide-induced macrophage functions. We also studied the role of AFB2, if any, in regulating nitric oxide-induced phagocytosis and intracellular killing by macrophages. So we addressed the role of sub-cytotoxic concentrations of AFB2 in regulating macrophage mediated nitric oxide synthesis with the resultant changes of mitochondrial membrane potential of macrophages and intracellular killing against infection by Candida albicans which is a virulent pathogen in humans in general and women in particular. This work may trigger a hectic pace of research activity for drug development against AFB2-induced immunosuppression which has remained hitherto elusive. We report in this paper that the exposure of aflatoxinB2 to macrophages has remarkably interfered with nitric oxide production and mitochondrial events in these macrophages, as is true with several carcinogenic compounds, causing the prevention of intracellular killing of Candida albicans.
2. Materials and Methods
2.1. Serum
- Serum was taken from blood samples of 3-month old rats (Norvegicus strain).
2.2. Yeast Cell Suspension
- Cell suspension of Candida albicans was made in RPM1 1640 medium and was opsonized with homologous serum for 30 minutes at 37°C. The final concentration of yeast cells was adjusted to 1.4 X 106 /ml.
2.3. Macrophage Collection
- Peritoneal cells were collected by flushing the peritoneal cavity of rats with 0.5μl/ml sterile heparin (0.85%) saline solution. Peritoneal cells were repeatedly washed, centrifuged at 1500 X g at 10°C for 20 min and the cell pellet was resuspended in RPM1 1640 medium supplemented with 5% fetal calf serum at a concentration of 1 X 106 /ml.
2.4. Nitric Oxide Production
- Peritoneal macrophage cell suspensions were added to each well of tissue culture chamber slide and were allowed to adhere to the slide for one hour at 37°C in 5% CO2 atmosphere. After incubation the cells were extensively washed with medium to remove non-adherent cells. Differential staining shows that >99% of the peritoneal suspension cells were macrophages. The adherent monolayer cells were resuspended in fresh medium and the concentration of macrophages was adjusted to 1 X 106 cells /ml and was activated with interferon-γ (INF-γ) at 40 units/ml (Sigma Co.) and incubated in 5% CO2 atmosphere at 37°C. Control culture of nonactivated macrophage (no IFN-γ) was exposed to media alone. In parallel experiments, NG-monomethy L-arginine (L-NMMA), an inhibitor of iNOS, was added to the culture at 500 μmol/l concentration. After 72 hours, macrophages were suspended in yeast cell suspension of Candida albicans in serum and incubated in humidified 5% CO2 atmosphere for one hour at 37°C. Then the supernatant, in triplicates, were removed for NO2 assay (an indicator of NO production) by the Griess reaction[15]. In brief, 50ml culture samples were combined in plate with a 1:1 mixture of 1% sulfanilamide in 2.5% H3PO4 and 0.1% napthylethelenediamide in 2.5% H3PO4. Plates were incubated at room temperature for 10 minutes and absorbance was determined at 550 nm. NO2 concentration was measured in triplicates using a standard curve of sodium nitrite from 1-125 μmol/l prepared in culture medium.
2.5. Intracellular Killing Assay
- Phagocytosing macrophages with intracellularly killed C.albicans (400 macrophages/slide) were stained with 15 ppm acridine orange solution for 90sec and counter-stained for 40 sec with crystal violet (1mg/ml, to quench fluorescence of extracellular C. albicans) and counted by fluorescence microscopy at 1000X magnification[16]. The dead Candida albicans fluoresced reddish yellow or orange, whereas live Candida albicans stained green. Differential count of macrophages containing C. albicans and their intracellular killing activity was expressed as the percentage of macrophages containing dead C. albicans[17]. All experiments were performed in triplicates.
2.6. Aflatoxin B2 Activity on NO Production
- In order to determine whether AFB2 activity could modulate NO production and intracellular killing, the macrophages were plated out as described above and after washing, the adherent cells were resuspended in the fresh RPM11640 medium. AFB2 (HiMedia) in the sub-cytotoxic concentrations of 0.0001, 0.001 and 0.01 µg/ml[18], was added to the macrophages in the medium, 72 hours before and 72 hours after the addition of IFN-γ (40 units /ml), as well as simultaneously with IFN-γ additions. The culture was incubated in 5% CO2 atmosphere at 37°C. The macrophages were suspended in yeast cell suspension, incubated and the NO production, as well as intracellular killings were determined as described above. A control set containing no AFB2 was observed.
2.7. Mitochondrial Membrane Potential Assay
- Changes in mitochondrial membrane potential was assessed with DiOC6[19] by flowcytometry of macrophages on a FACS scan flowcytometer (Becton Dickinson, USA), using CellQuest software.
2.8. Cytotoxicity Test
- Viable cells were counted using Trypan Blue Exclusion Test. It is based on the principle that live cells possess intact cell membranes that exclude trypan blue, whereas dead cells do not. The macrophage cell suspension was mixed with the dye and visually examined to determine dye taking up. The viable cells showed clear cytoplasm whereas the nonviable ones showed blue cytoplasm. An aliquot of cell suspension was centrifuged for 5min at 100X g and the supernatant was discarded for testing viability. The cell pellet was resuspended in 1ml PBS medium, mixed (1:1) with 0.4% Trypan blue and allowed to incubate for 3 minutes at room temperature. The concentration of viable macrophage cells was examined by haemocytometry. This percentage of viable cells per ml of aliquot = total number of viable macrophages per ml of aliquot / total number of cells per ml of aliquot.
3. Results
3.1. NO Production and Intracellular Killing by Rat Peritoneal Macrophages
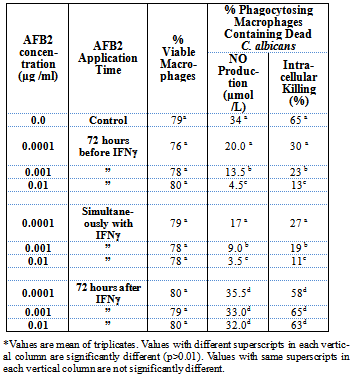
- Peritoneal macrophages were able to synthesize NO and kill intracellular C. albicans when activated with IFN-γ. Nevertheless, NO synthesis and microbicidal activity were inhibited in the presence of L-NMMA, an inhibitor of iNOS, which indicates that the intracellular killing of C. albicans by macrophages is mediated by NO (Table 1).
3.2. AFB2 Prevents NO Synthesis & NO-Mediated Immuno-Functions of Macrophages, viz., Intracellular Killing
- Sub-cytotoxic doses of AFB2 when added to the culture 72 hours before IFN-γ, or simultaneously with IFN-γ, significantly inhibited the synthesis of NO and intracellular killing of C. albicans by the macrophages despite the presence of the activating signal IFN-γ (Table 2). It appeared that AFB2 affected the macrophages in such a way that the macrophage either did not receive or could not act upon the activating signals. Also, it seemed possible that AFB2 might have affected the signalling pathways.
|
|
3.3. Mitochondrial Events in NO Induced Macrophages and its Prevention by AFB2
- It has been assumed that mitochondria are involved in NO mediated immune functions of macrophage(ø). Reduction of mitochondrial transmembrane potential (ѱm) precedes NO induction[20]. This type of mitochondrial change in NO-induced macrophage functions has not yet been reported.To detect whether AFB2 is affecting mitochondrial signalling in NO mediated macrophage functions, we have investigated mitochondrial membrane potential of NO induced and interferon-γ stimulated macrophage. We observed that the number of macrophage cells with reduced mitochondrial membrane potential remarkably increased when the macrophages were activated with only NO production (Fig. 1). Such NO mediated increase in membrane potential was completely prevented in macrophages, when stimulated by sub-cytotoxic concentrations of AFB2. These findings were corroborated by previous results of remotely related work[21]. Thus the present research established strong evidence for the role of sub-cytotoxic concentrations of AFB2 in mitochondrial changes in NO-induced macrophages activated by IFN-γ.
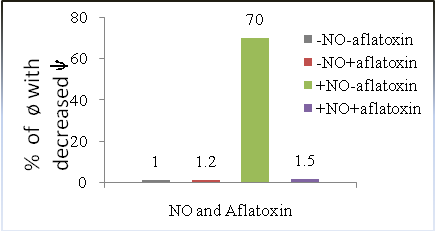 | Figure 1. Mitochondrial Events in NO Induced Macrophage (ø) and its Prevention by Sub-cytotoxic Level of AFB2 |
4. Discussion
- Aflatoxin B2 has been known to cause carcinogenesis and other diseases. In addition, AFB2 has been found to be an immunotoxic secondary metabolite. However, the present work has provided compelling evidence that even sub-cytotoxic concentration of AFB2 can be immunosuppressive and can affect the intracellular killing of C. albicans by rat peritoneal macrophage. The effects of AFB2 on NO production were determined and the latter was assumed to be involved in the response of macrophage to AFB2. The present work reveals the effects of sub-cytotoxic concentrations of AFB2 on the immune functions of macrophage which may cause exposed people susceptible to fungal infections. Intracellular environment of macrophage cells, which is essential to innate host defenses against invading microorganisms, may however provide a refuge for C. albicans-survival and dissemination in presence of AFB2 (sub-cytotoxic) exposure. In tune with this rationale, we presumed that C. albicans might have induced self-protective mechanism(s) by reducing both the increased NO production and the increased mitochondrial membrane potential inside macrophages by the activation of sub-cytotoxic AFB2 as it happens with some other obligate intracellular microorganisms. If this presumption stands valid, the present experiment may argue that C.albicans is capable of inducing self-protective effect and AFB2 induced effect in macrophage, thus helping the macrophage to allow the inner pathogens to replicate and persist while being invisible to immune system[17,22]. It is established that some pathogens such as Legionella pneumophila, Chlamydiae spp., and some other microbes follow this process. The present study has also shown that C.albicans can live inside macrophages without affecting the viability of the latter. All these observed phenomena indicate that C.albicans might also be capable of protecting macrophages from changes of mitochondrial membrane potential-induced apoptosis.The impaired intracellular killing of C.albicans by macrophages may plausibly be due to the non-activity of iNOS with the resultant suppression of NO production. Presently, work on protection against aflatoxin toxicity is on the rise[23] but insufficient work has been done in this direction.
ACKNOWLEDGEMENTS
- We gratefully acknowledge the Department of Food Technology & Biochemical Engineering, Jadavpur University, Kolkata, for providing necessary facilities.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML