-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Life Sciences
p-ISSN: 2163-1387 e-ISSN: 2163-1395
2012; 2(2): 21-28
doi: 10.5923/j.als.20120202.05
Genotoxic and Antigenotoxic Properties of Calendula officinalis Extracts in Mice Treated with Methyl Methanesulfonate
Daniela Dimer Leffa 1, Raquel da Rosa 1, Bruna Pazini Munhoz 1, Andréia de Araújo Martins Mello 1, Fernanda Dagostim Mandelli 2, Patrícia de Aguiar Amaral 2, Ângela Erna Rossatto 2, Vanessa Moraes de Andrade 2
1Laboratório de Biologia Celular e Molecular (LABIM), Universidade do Extremo Sul Catarinense (UNESC), Criciúma, Santa Catarina State, 3167, Brasil
2Laboratório de Estudos Etnofarmacológicos (G-FITO), Universidade do Extremo Sul Catarinense (UNESC), Criciúma, Santa Catarina State, 3167, Brasil
Correspondence to: Vanessa Moraes de Andrade , Laboratório de Estudos Etnofarmacológicos (G-FITO), Universidade do Extremo Sul Catarinense (UNESC), Criciúma, Santa Catarina State, 3167, Brasil.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Calendula officinalis L. (Asteraceae) is used in the traditional medicine for centuries to treat several diseases. The aim of the present study was to investigate in vivo the genotoxicity/mutagenicity and antigenotoxicity/antimutagenicity potential of the Calendula officinalis L. The CF-1 male mice were treated with ethanolic (250 or 500 mg/Kg) or aqueous (90 mg/Kg) extracts of C. officinalis for 2 weeks prior to treatment with saline or methyl methanesulfonate. No genotoxic or mutagenic effect was observed at the doses of ethanolic and aqueous extracts of C. officinalis in blood and bone marrow samples from animals after 2 weeks of treatment, analyzed by the Comet assay and Micronucleus test, respectively. In addition, ethanolic extracts in both doses (250 or 500 mg/Kg) and aqueous extracts (90 mg/Kg) of this plant showed an antigenotoxic effect by Comet assay, repairing the DNA damage caused by MMS. At the Micronucleus test only aqueous extract of C. officinalis revealed a protector effect to genetic material. These results suggest that all the extracts of C. officinalis contained protective substances that decreasing damage to genetic material. Despite this protective or antigenotoxic effect from this plant, it should be used with caution by the population.
Keywords: Calendula Officinalis, Comet Assay, Micronucleus Test, Genotoxicity, Antigenotoxicity
Article Outline
1. Introduction
- The search of natural products for cancer therapy represents an area of great interest in which plants had been the most important source. Furthermore, the study of plant compounds origin has generated great interest in the fields of food and medicine since many phytochemicals with different pharmacological properties have shown responses for the prevention or treatment of different diseases[1].The correct use of plants by the general population requires the use of medicinal plants selected for their efficacy and safety, based on folk tradition or validated scientifically[2]. The use of herbal infusions to cure different types of diseases is very common in Brazilian folk medicine, and frequently replaces modern medicine. The diversity of plant species in Brazil is a potential source of biologically active compounds whose effects on human health or in the genetic material are often unknown. However, not all species are harmless to human health, and some may present toxic and/or mutagenic substances in their phytochemical composition[3-5]. On the other hand, a protective action of phytochemical compounds on genetic material has been reported, leading to its repair or to preserving its integrity[4; 6; 7].The search for bioactive products those are both effective and non-toxic in the prevention and/or treatment of cancers and other diseases is an important research line. In this manner, Calendula officinalis L. (Asteraceae), also known as marigold, is an aromatic plant with yellow or orange flowers that are used to make herbal preparations. This annual herb native to the Mediterranean region is cultivated in Europe and America for ornamental and medicinal purposes and has been used in traditional medicine for centuries to treat several internal and external inflammatory conditions, gastric and duodenal ulcers and haemorrhoids[8].Its beneficial activity is related to the content of various secondary metabolites such flavonoids (including lutein, quercetin, protocatechuic acid, etc.), triterpenoids (including faradiol, oleanolic acid, beta-amyrin, calenduladiol, etc.), and the alkaloid narcissi[9]. Flowers also are rich in carotenoids of which flavoxanthin has been reported to be present at 28.5% of total carotenoids followed by luteoxanthin[10]. Flowers are also found to contain lycopene and b-carotene. Coumarins are also an active ingredient in Calendula officinalis[11].In the last decade, there was increased interest in the antioxidant properties of C. officinalis extracts (infusions and tinctures) because of its rather high polyphenol and carotenoid content[11-13]. However, the protective effect of C. officinalis extracts against DNA damage has just studied in vivo in one study[13].The antitumoral and antimutagenic properties of some components of C. officinalis have been described[15; Jiménez-Medina et al. 2006; 13). Jiménez-Medina et al. (2006) have studied the aqueous extract of C. offinalis in order to measure its anti-tumor and immunomodulatory activities in vitro. They found that the extract showed a potent inhibition of tumor cell proliferation when tested on a wide variety of human and murine tumor cell lines. In relation to antigenotoxic studies, Pérez-Carreón et al.[15] have analyzed whether C. officinalis extracts induce unscheduled DNA synthesis (UDS) in rat liver cell cultures, and if these extracts can reverse diethylnitrosamine (DEN)-induced UDS. They found that both aqueous and ethanolic extract showed complete reversion of the DEN effect. In another work, Frankic et al.[13] evaluated the protective effect of C. officinalis propylene glycol extracts against oxidative DNA damage and lipid peroxidation induced by high polyunsaturated fatty acid (PUFA) intake in young growing pigs. The results showed an effective DNA protection from oxidative damage induced by PUFA. Nevertheless, a genotoxic effect has been reported of a fluid extract at concentrations from 0.1 to 1.0 mg of solid/mL in the mitotic segregation assay of the heterozygous diploid D-30 of Aspergillus nidulans although the same extracts were not mutagenic in the Salmonella/microsome assay at concentrations of 50 to 5000 µg/plate (± S9), and in the mouse bone marrow cells micronucleus test, where the extract was dosed orally up to 1g/kg for two days were not genotoxic either[16].Culturally, the consumption of C. officinalis in Brazil is by means of the teas and infusions, however the use of C. officinalis by oral route in humans needs a safety evaluation for this route of administration. Available data are insufficient to support the safety of C. officinalis extract by oral route. Thus, considering the strong therapeutic use of C. officinalis, this study aimed to investigate the mutagenic/antimutagenic and genotoxic/antigenotoxic activity of aqueous and ethanolic extracts of C. officinalis utilizing the Micronucleus Test and the Comet assay.
2. Material and Method
2.1. Identification of Plant Material and Extract Preparation
- Aerial parts of C. officinalis, family Asteraceae, were collected in , SC, Brazil in September voucher specimen (number CRI 7379) of C. officinalis was deposited at Herbarium Pe. Dr. Raulino Reitz, Universidade do Extremo Sul Catarinense, Criciúma, SC, Brazil. The aerial parts were allowed to dry under air circulation () for 3 days. The ethanolic extract was obtained according to the methodology proposed by Farmacopéia Brasileira[17]. The ethanolic extract was prepared by soaking 200g of the dried pharmacogen (ground in a knife mill) in 1000 mL of solvent (water-alcohol solution of ethanol 45%) for about 21 days at room temperature, protected from light, with occasional agitation (once a day) and no renewal of liquid extractor. After, the liquid was strained with the aid of gauze and then filtered on filter paper, and completed the volume to 1000 mL with the same solvent. Then, the extract was evaporated to dryness under reduced pressure. This dry extract was then diluted in water to give two different doses to be tested (250 and 500 mg/kg, adapted by Lagarto et al.[18]. The aqueous extract was obtained by infusion of of dry pharmacogen in 20mL of distilled hot water, just before use. The dose of the aqueous extract (90mg/kg) was based on the calculation of total solids.
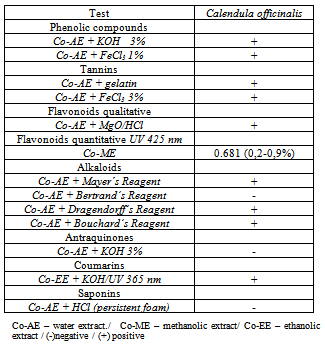
2.2. Phytochemical Screening
- The phytochemical analysis (flavonoids, tannins, anthraquinones, alkaloids, saponins, coumarins and cardiac glycosides) of the aerial parts of C. officinalis was carried out according to the methods described by Harborn[19]. The thin layer chromatography analyses were performed following systems and developers indicated by Wagner and Bladt[20], and the aluminum chloride colorimetric method was used for flavonoids quantitative determination[17]. Each plant extracts (0.5 mL of 1:10 g) in methanol were separately mixed with 1.5 mL of methanol, 0.5 mL of 2% aluminum chloride, 0.1 mL of potassium acetate and 2.8 mL of distilled water. It remained at room temperature for 30 min; the absorbance of the reaction mixture was measured at 425nm with biospectro Model SP-22 UV/Visible spectrophotometer.
2.3. Animals and Treatments
- CF-1 male mice (weighing 30 - 45g) were obtained from the breeding colonies of the Universidade do Extremo Sul Catarinense, UNESC, . The animals were kept in plastic cages in an experimental room under controlled conditions of temperature (22 ± ), humidity (55 ± 10%), 12-h light/dark cycle and with ad libitum access to diet and water. The animals were randomized at the beginning of the experiment. The study design was approved by the Animal Ethical Committe of UNESC (protocol number 043/2008) and the experiments were conducted in accordance with the ethical principles of the Brazilian - COBEA.Methyl methanesulfonate (MMS; Sigma-Aldrich), a monofunctional sulfurcontaining compound commonly used as a solvent and as a catalyst for polymerization, alkylation and esterification reactions[21], was used to induce mutations and DNA damage for the antimutagenic/antigenotoxic evaluations. MMS was diluted in 0.9% NaCl just before use. The route of exposure was by intraperitoneal (ip) injection of 40 mg/kg b.w.[22].
2.4. Experimental Design
- The evaluation of DNA damage or protection by the aqueous and ethanolic extracts of C. officinalis were performed according the protocol developed by Azevedo et al.[23] with adaptations as follows (see Figure 1): Initially, the mice were divided into 7 groups, with 5 animals per group. In group 1, mice received only distilled water (10 mL/kg b.w. per day by gavage) for 14 days prior to treatment with 0.9% NaCl by intraperitoneal (i.p) injection. Group 2 also received distilled water (10 mL/kg b.w. per day by gavage) for 14 days, but the mice were treated on day 15 with MMS (40 mg/kg b.w.) by i.p. injection. Groups 3 and 4 received ethanolic extract of C. officinalis administered orally (10 mL/kg b.w. per day by gavage) prepared as two different doses: 250 mg/kg (group 3) and 500 mg/kg (group 4), for 14 days prior to treatment with MMS. Group 5 received only ethanolic extract of C. officinalis administered orally (500 mg/kg) for 14 days prior to a 0.9% NaCl i.p. injection on day 15. Groups 6 and 7 received the aqueous extract of C. officinalis (90 mg/kg) (10 mL/kg b.w. per day by gavage) for 14 days prior to a 0.9% NaCl or MMS (40mg/kg) i.p. injection on day 15. The mice were killed by cervical dislocation, 24h after treatment, for the evaluation of micronucleated polychromatic erythrocytes (MNPCEs) in the bone marrow. The Comet assay was performed on all groups and samples of peripheral blood were collected from mouse tail tips by means of a small incision 4h after treatment with MMS.
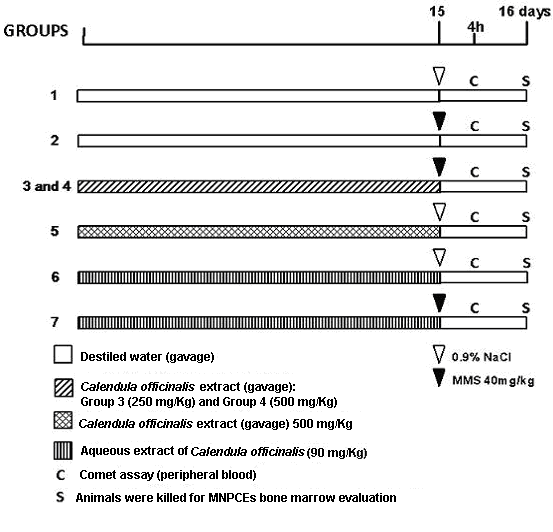 | Figure 1. Study trial to test the antimutagenicity and antigenotoxicity of Calendula officinalis extract on the mutagen-induced micronulei and comet assay effects |
2.5. Micronucleus Test
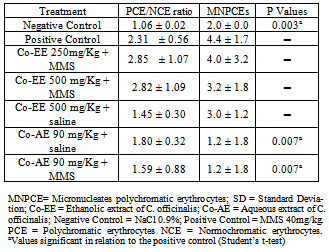
- The micronucleus assay was performed according to the US Environmental Protection Agency Gene-Tox Program[24]. Bone marrow from both femurs was suspended in foetal calf serum and smears on clean glass slides were prepared. Slides were air-dried, fixed in methanol, stained in 10% Giemsa and coded for a blind analysis. Two thousand polychromatic erythrocytes were analyzed per mouse. To detect possible cytotoxic effects, the proportion of PCE and NCE (normochromatic erythrocytes) in 200 erythrocytes/animal was calculated. The slides were scored blindly using a light microscope with a 100x immersion objective.
2.6. Comet Assay
- Single Cell Gel Electrophoresis or Comet assay is a highly sensitive method for the assessment of DNA damage formation and repair both at clinically relevant and low doses. The alkaline Comet assay was performed as described by Singh et al.[25]. Peripheral blood samples were collected by the tail from each animal in heparinized microtubes 4 h after the MMS treatment. Briefly, 5µL of whole blood was embedded in a layer consisting of 95 ųL of 0.75% low melting point agarose gel on frosted slides and immersed in a lysis buffer (2.5M NaCl, 100mM EDTA, and Tris [pH 10.0–10.5] with freshly added 1% Triton X-100 and 10% dimethyl sulfoxide) for a minimum of 1 h and a maximum of 1 week. Subsequently, the slides were incubated in freshly made alkaline buffer ( NaOH and EDTA, pH>13) for 20 min. The nuclei were electrophoresed for 20 min at 25 V (0.90 V/cm) and 300 mA, and then the alkali was neutralized with 0.4M Tris (pH 7.5). After neutralization, the slides were fixed (15% w/v trichloroacetic acid, 5% w/v zinc sulfate, 5% glycerol), washed in distilled water and dried overnight. The gels were re-hydrated for 5 min in distilled water, and then stained for 15 min (37˚C) with a solution containing the following sequence: 34 mL of Solution B ( 0.2% w/v ammonium nitrate, 0.2% w/v silver nitrate, 0.5% w/v tungstosilicic acid, 0.15% v/v formaldehyde, 5% w/v sodium carbonate) and 66 mL of Solution A (5% sodium carbonate). The staining was stopped with 1% acetic acid and then the gels were air-dried[26]. The extent of the DNA damage was assessed using the Collins’ visual classification method[27]. Cells were scored from 0 (undamaged) to 4 (maximally damaged) according to tail intensity (size and shape), resulting in a single DNA damage score (damage index) for each sample and, consequently, for each group. Thus, a damage index (DI) of the group could range from 0 (completely undamaged) 100 cells × 0) to 400 (maximum damage) 100 cells (× 4). The percentage damage frequency (DF) was calculated for each sample on the basis of the number of cells with a tail versus with no tail.
2.7. Statistical Analysis
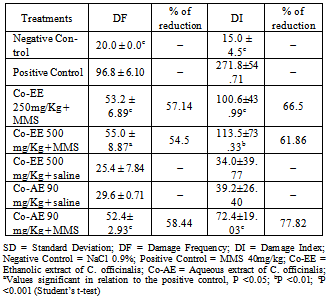
- The normality of variables was assessed using the Kolmogorov-Smirnov test. For the micronucleus test and Comet assay, we performed multiple pair-wise comparisons between the experimental groups and the positive and negative controls using Student’s t-test at a significance level of 0.05. The statistical package used was BioEstat 5.0. The percentage of reduction in the frequency of MMS induced DNA damage was calculated according to Waters et al.[28] and Azevedo et al.[29].
3. Results and Discussion
- Table 1 shows the phytochemical analyses of C. officinalis extract. The analyses indicated the presence of fenolic compounds, tannins, flavonoids, alkaloids and coumarins. Yet, other secondary metabolites such as anthraquinones and saponins were not detected.
|
|
4. Conclusions
- We can conclude that C. officinalis extracts proposed for internal use in traditional medicine protects the organism against DNA damage. Taken together, it can be inferred so that aqueous or ethanolic extract of C. officinalis are endowed with antigenotoxic properties, and its use in pre-treatment diminishes the induction of DNA damage by an alkylant agent.
ACKNOWLEDGEMENTS
- This work was supported by UNESC (Universidade do Extremo Sul Catarinense) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico).
References
| [1] | Jiménez-Medina, E., Garcia-Lora, A., Paco, L., Algarra, I., Collado, A., Garrido, F. (2006). A new extract of the plant Calendula of oficinalis produces a dual in vitro effect: Cytotoxic anti-tumor activity and lymphocyte activation. BMC Cancer (6): 119 |
| [2] | Tovart, R.T. (2009). Clinical approach to clinical herbal toxicity. Seminars in Diagnostic Pathology (26): 28-37 |
| [3] | Bresolin, S., Vargas, V.M.F. (1993). Mutagenic potencies of medicinal plants screened in the ames test. Phytotherapy Research (7): 260-262 |
| [4] | Fernandes, J.B.F., Vargas, V.M.F. (2003). Mutagenic and antimutagenic potential of the medicinal plants M. laevigata and C. xanthocarpa. Phytotherapy Research (17): 269-273 |
| [5] | Sá-Ferreira, I.C.F., Vargas, V.M.F. (1999). Mutagenicity of medicinal plant extracts in Salmonella/microsome assay. Phytotherapy Research (13): 397-400 |
| [6] | Berhow, M., Wagner, E., Vaughn, S., Plewa, M. (2000). Characterization and antimutagenic activity of soybean saponins. Mutation Research (448): 11-22 |
| [7] | Souza, A.C., Alviano, D.S., Alves, P.B., Alviano, C.S., Gattass, C.R. (2004). Melissa officinalis L. essential oil: antitumoral and antioxidant activities. Journal of Pharmacy and Pharmacology (56): 677-681 |
| [8] | Fiume, M.Z. (2001). Final Report on the safety assessment of Calendula Officinalis extract and Calendula Officinalis. International Journal of Toxicology (20): 13-20 |
| [9] | Matysik, G., Wojciak-Kosior, M., Paduch, R. (2005). The influence of Calendula officinalis flos extract on cell cultures and the chromatographic analysis of extracts. Journal of Pharmaceutical and Biomedical Analysis (38): 285–292 |
| [10] | Kishimoto, S., Maoka, T., Sumitomo, K., Ohmiya, A. (2005). Analysis of carotenoid composition in petals of calendula (Calendula officinalis L.). Bioscience, Biotechnology, and Biochemistry (69): 2122–2128 |
| [11] | Preethi, K.C., Kuttan, G., Kuttan, R. (2006). Antioxidant potential of an extract of Calendula officinalis flowers in vitro and in vivo. Pharmaceutical Biology (44): 691–697 |
| [12] | Ćetković, G.S., Djilas, S.M., Canadanovic-Brunet, J.M., Tumbas, V.T. (2004). Antioxidant properties of marigold extracts. Food Research International (37): 643–650 |
| [13] | Fonseca, Y.M., Catini, C.D., Vicentini, F.T.M.C., Nomizo, A., Gerlach, R.F., Fonseca, M.J.V. (2010). Protective effect of Calendula officinalis extract against UVB-induced oxidative stress in skin: Evaluation of reduced glutathione levels and matrix metalloproteinase secretion. Journal of Ethnopharmacology (127): 596–601 |
| [14] | Frankic, T., Salobir, K., Salobir, J. (2008). The comparison of in vivo antigenotoxic and antioxidative capacity of two propylene glycol extracts of Calendula officinalis (marigold) and vitamin E in young growing pigs. Journal of Animal Physiology and Animal Nutrition (41): 1-7 |
| [15] | Pérez-Carreón, J.I., Cruz-Jiménez, G., Licea-Veja, J.A., Popoca, E.A., Fazenda, S.F., Villa-treviño, S. (2002). Genotoxic and anti-genotoxic properties of Calendula officinalis extracts in rat liver cell cultures trated with diethylnitrosamine. Toxicology in Vitro (16): 253-258 |
| [16] | Ramos, A., Edreira, A., Vizoso, A., Betancourt, J., Lopez, M., Decalo, M. (1998). Genotoxicity of an extract of Calendula officinalis L. Journal of Ethnopharmacology (61): 49–55 |
| [17] | Farmacopéia Brasileira. (2001). Farmacopéia Brasileira. Atheneu, São Paulo. pp. 1320 |
| [18] | Lagarto, A., Bueno, V., Guerra, I., Valdés, O., Veja, Y., Torres, L. (2011). Acute and subchronic oral toxicities of Calendula officinalis extract in Wistar rats. Experimental and Toxicologic Pathology (63): 387-391 |
| [19] | Harborn, J.B. (1998). Phenolic compounds. In: Harborne, J.B. (Ed), Phytochemical methods: a guide to modern techniques of plant analysis. Chapman & Hall; United Kingdom. UK. pp. 40-106 |
| [20] | Wagner, H., Bladt, S. (2009). Plant Drug Analysis: A Thin Layer Chromatography Atlas. Springer, Germany. pp. 384 |
| [21] | Vrzoc, M., Petras, M.L. (1997). Comparison of alkaline single cell gel (Comet) and peripheral blood micronucleus assays in detecting DNA damage caused by direct and indirect acting mutagens. Mutation Research (381): 31-40 |
| [22] | Pereira, P., Tysca, D., Oliveira, P., da Silva, B.L.F., Picada, J.N., Ardenghi, P. (2005). Neurobehavioral and genotoxic aspects of rosmarinic acid. Pharmacological Research (52): 199-203 |
| [23] | Azevedo, L., Lima, P.L.A., Gomes, J.C., Stringheta, P.C., Ribeiro, D.A., Salvadori, D.M.F. (2007). Differential response related to genotoxicity between eggplant (Solanum melanogena) skin aqueous extract and its main purified anthocyanin (delphinidin) in vivo. Food and Chemical Toxicology (45): 852- 858 |
| [24] | Mavournin, K.H., Blakey, D.H., Cimino, M.C., Salamone, M.F., Heddle, J.A. (1990). The in vivo micronucleus assay in mammalian bone marrow and peripheral blood. A report of the U.S. Environmental Protection Agency Gene-Tox Program. Mutatation Research (232): 29-80 |
| [25] | Singh, N.P., McCoy, M.T., Tice, R.R., Schneider, E.L. (1988). A simple technique for quantification of low levels of DNA damage in individual cells. Experimental Cell Research (175): 184-191 |
| [26] | Villela, I.V., Oliveira, L.M.O., Silva, J., Henriques, J.A.P. (2006). DNA damage and repair in haemolymph cells of golden mussel (Limnoperna fortunei) exposed to environmental contaminants. Mutation Research (605): 78-86 |
| [27] | Collins, A., Dusinska, M., Franklin, M., Somorosyska, M., Petroyska, H., Duthie, S., Fillion, L., Panyotidis, M., Raslová, K., Vaughan, N. (1997). Comet assay in human biomonitoring studies: reliability, validation, and applications. Environmental and Molecular Mutagenesis (30): 139-146 |
| [28] | Waters, M.D., Brady, A.L., Stack, H.F., Brockman, H.E. (1990). Antimutagenicity profiles for some model compounds. Mutation Research (238): 57-85 |
| [29] | Azevedo, L., Gomes, J.C., Stringheta, P.C., Gontijo, A.M.M.C., Padovani, C.R., Ribeiro, L.R., Salvadori, D.M.F. (2003). Black bean (Phaseolus vulgaris L.) as a protective agent against DNA damage in mice. Food and Chemical Toxicology (41): 1671-1676 |
| [30] | Brown, D.J., Dattner, A.M. (1998). Phytotherapeutic approaches to common dermatologic conditions. Archives of Dermatology (134): 1401–1404 |
| [31] | Della-Loggia, R., Tubaro, A., Sosa, S., Becker, H., Saar, S., Issac, O. (1994). The role of triterpenoids in the topical anti-inflammatory activity of Calendula officinalis flowers. Plant Medicine (60): 516–520 |
| [32] | Dumenil, G., Chemli, R., Balausard, G. (1980). Evaluation of antibacterial properties of Calendula officinalis flowers and mother homeopathic tinctures of Calendula officinalis. Annales Pharmaceutiques Fran (38): 493–499 |
| [33] | Kasiram, K., Sakharkar, P.R., Patil, A.T. (2000). Antifungal activity of Calendula officinalis. Indian Journal of Pharmaceutical Sciences (6): 464–466 |
| [34] | Barbour, E.K., Sagherian, V., Talhouk, S., Talhouk, R., Farran, M.T., Sleiman, F.T., Harakeh, S. (2004). Evaluation of homeopathy in broiler chickens exposed to live viral vaccines and dministered Calendula officinalis extract. Medical Science Monitor (10): 281–285 |
| [35] | Boucaud-Maitre, Y., Algernon, O., Raynaud, J. (1988). Cytotoxic and antitumoral activity of Calendula officinalis extracts. Pharmazie (43): 220–221 |
| [36] | Khalil, M.Y., Moustafa, A.A., Naguib, N.Y. (2007). Growth, phenolic compounds and antioxidant activity of some medicinal plants grown under organic farming conditions. World Journal of Agricultural Sciences (3): 451–457 |
| [37] | Laughton, M.J., Halliwell, B., Evans, P.J., Hoult, J.R. (1989). Antioxidant and pro-oxidant actions of plant phenolics quercetin, gossypol and myricetin. Effects on lipid peroxidation, hidroxil radical generation and bleomycin dependant DNA damage. Biochemical Pharmacology (38): 2859–2865 |
| [38] | Duarte-Silva, I., Gaspar, J., Gomes-da-Costa, G., Rodrıguez, A.S., Laires, A., Rueff, J. (2000). Chemical features of flavonols effecting their genotoxicity. Potential implications in their use as therapeutical agents. Chemico-Biological Interactions (124): 29–51 |
| [39] | Bakkali, F., Averbeck, S., Averbeck, D., Zhiri, A., Idaomar, M. (2005). Cytotoxicity and gene induction by some essential oils in the yeast Saccharomyces cerevisiae. Mutation Research (585): 1-13 |
| [40] | Re, T.A., Mooney, D., Antignac, E., Dufour, E., Bark, I., Srinivasan, V., Nohynek, G. (2009). Application of the threshold of toxicological concern approach for the safety evaluation of calendula flower (Calendula officinalis) petals and extracts used in cosmetic and personal care products. Food and Chemical Toxicology (47): 1246–1254 |
| [41] | Barajas-Farias, L.M., Pérez-Carreón, J.I., Arce-Popoca, E., Fattel-Fazenda, S., Alemán-Lazarini, L., Hernandez-García, S., Salcido-Neyoy, M., Cruz-Jime nez, F.G., Camacho, J., Villa-Trevinó, S. (2006). A dual and opposite effect of Calendula officinalis flower extract: chemoprotector and promoter in a rat hepatocarcinogenesis model. Plant Medicine (72): 217–221 |
| [42] | Gladine, C., Morand, C., Rock, E., Bauchart, D., Durand, D. (2007). Plant extracts rich in polyphenols (PERP) are efficient antioxidants to prevent lipoperoxidation in plasma lipids from animals fed n-3 PUFA supplemented diets. Animal Feed Science and Technology (136): 281–296 |
| [43] | Preethi, K.C., Kuttan, R. (2008). Effect of Calendula officinalis Flower Extract on Acute Phase Proteins, Antioxidant Defense Mechanism and Granuloma Formation During Thermal Burns. Journal of Clinical Biochemistry and Nutrition (43): 58–64 |
| [44] | Palmer, H.J., Paulson, E.K. (1997). Reactive oxygen species and antioxidants in signal transduction and gene expression. Nutrition Reviews (55): 353–361 |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML