-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Life Sciences
p-ISSN: 2163-1387 e-ISSN: 2163-1395
2012; 2(2): 5-8
doi: 10.5923/j.als.20120202.02
Antibacterial Activities of Azadirachta Indica against Some Bacterial Pathogens
Abalaka M. , Oyewole O. A. , Kolawole A. R.
Department of Microbiology, Federal University of Technology, PMB 65, Minna, Niger State, Nigeria
Correspondence to: Oyewole O. A. , Department of Microbiology, Federal University of Technology, PMB 65, Minna, Niger State, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The antibacterial effect of Azadirachta indica against Pseudomonas aeruginosa, Klebsiella ozanae, Staphylococcus aureus and Escherichia coli was determined using the agar cup plate technique. The phytochemical components of A. indica showed the presence of saponin and phlobatanin and the absence of alkaloids, tannins, phenolics, glycosides, flavonoids and triterpenes. The result showed that the test organisms were susceptible to 500mg/ml, 50mg/ml and 5mg/ml of the plant extract. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were determined. The result showed that the MIC and MBC were 5mg and 50mg respectively for Pseudomonas aeruginosa, Kl. ozanae, Staphylococcus aureus and Escherichia coli. The result of the study suggests that extracts of A. indica could be suitable for the treatment of various infections caused by P. aeruginosa, K. ozanae, S. aureus and E. coli.
Keywords: Antibacterial, Azadirachta Indica, Phytochemical, Minimum Inhibitory Concentration, Minimum Bacteri Cidal Concentration
1. Introduction
- Plants contain many biologically active compounds which have potential for development as medicinal agents. Herbal medicines already form the basis of therapeutic use in the developing countries, but of recent, there has been an increase in the use of herbal medicines in the developed world too (De and Ifeoma, 2002; El-Mahmood et al., 2010). Plants provide an alternative strategy in search for new drugs. There is a rich abundance of plants reputed in traditional medicine to possess protective and therapeutic properties (Kayode and Kayode, 2011). It is likely that plants will continue to be a valuable source of new molecules which may, after possible chemical manipulation, provide new and improved drugs (Shah et al., 2006). Bacterial resistance to antibiotics represents a serious problem for clinicians and the pharmaceutical industry and great efforts are being made to reverse this trend, and one of them is the widespread screening of medicinal plants from the traditional system of medicine hoping to get some newer, safer, and more effective agents that can be used to fight infectious diseases (Natarajan et al., 2003). Azadirachta indica is one of such medicinal plants belonging to the Meliaceae family and is indigenous to southern Asia (Akula et al., 2003). Azadirachta indica, commonly known as neem, has attracted worldwide prominence in recent years, owing to its wide range of medicinal properties. Neem has been extensively used in Ayurveda, Unani and Homoeopathic medicine and has become a cynosure of modern medicine. Neem elaborates a vast array of biologically active compounds that are chemically diverse and structurally complex. More than 140 compounds have been isolated from different parts of neem. All parts of the neem tree- leaves, flowers, seeds, fruits, roots and bark have been used traditionally for the treatment of inflammation, infections, fever, skin diseases and dental disorders. The medicinal utilities have been described especially for neem leaf. Neem leaf and its constituents have been demonstrated to exhibit immunomodulatory, anti-inflammatory, antihyperglycaemic, antiulcer, antimalarial, antifungal, antibacterial, antiviral, antioxidant, antimutagenic and anticarcinogenic properties (Talwar et al., 1997; Biswas et al., 2002; Subapriya and Nagini, 2005).The objectives of this study therefore are to determine the phytochemical components of the leaf extracts of A. indica, to determine the minimum inhibitory concentration (MIC) of the extract on Pseudomonas aeruginosa, Klebsiella ozanae, Staphylococcus aureus and Escherichia coli and determine the minimum bactericidal concentration (MBC) of the extract on Pseudomonas aeruginosa, Klebsiella ozanae, Staphylococcus aureus and Escherichia coli.
2. Materials and Methods
- Collection and preparation of samplesThe bark of A. indica was collected from different locations in Minna metropolis. It was air-dried for six weeks in microbiology laboratory of Federal University of Technology, Minna. The dried materials were pulverised in mortar and packaged in bottles for analysis. Collection of specimenPure cultures of Pseudomonas aeruginosa, Klebsiella ozanae, Staphylococcus aureus and Escherichia coli were obtained from General Hospital Minna. Niger State and were subcultured in agar slants.Extraction of materialsEthanol and water were used as solvents for the extraction of the plant materials. One hundred and fifty gram (150g) of pulverised sample was suspended in 750ml of 75% ethanol for 120hours. The extracts were decanted, filtered and evaporated in vacuole at 45oC.Phytochemical Screening of Extracts of A. indicaThe phytochemical components of extracts of A. indica was determined using methods described by Odebiyi and Sofowora (1978) and Trease and Evans (1989). The phytochemical components analysed for were alkaloids, tannins, phenolics, glycosides, saponins, flavonoids, steroids, phlobatanins and triterpenes.Antimicrobial susceptibility testSusceptibility test of the test organisms to extracts of A. indica at concentrations of 500mg/ml, 50mg/ml and 5mg/ml was carried out using agar cup plate technique as described by Silver et al. (1997). Nutrient agar was sterilized using autoclave at 121oC for 15 minutes. It was then poured on to plates and allowed to solidify. Standardized inoculum of each test organisms was spread on to agar plates so as to achieve a confluent growth. The impregnated discs with different concentrations of the extract were placed on the surface of the medium at three points equidistant from one another. The plates were then incubated at 37oC for 24 hours.Determination of Minimum Inhibitory Concentration (MIC)The minimum inhibitory concentration (MIC) of the test organisms was determined using the tube dilution technique. Nine millilitre (9ml) of the nutrient broth was pippeted into various test tubes containing concentrations of 500mg/ml, 50mg/ml and 5mg/ml of the extract. The overnight culture of the test organisms diluted at 106cfu/ml was added to the test tubes and then incubated at 37oC for 24 hours. The least concentration of the extract that did not indicate any visible growth of the incubated organisms in broth culture was taken as the minimum inhibitory concentration (MIC) (Hugo and Russel, 1983).
3. Result
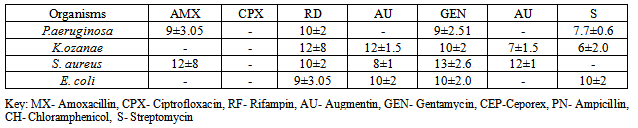
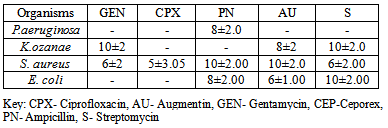
- Phytochemical screening of the extractsTable 1 shows the phytochemical screening of the extract of the A. indica. The phytochemical components of A. indica showed the presence of saponin and phlobatanin and the absence of alkaloids, tannins, phenolics, glycosides, flavonoids and triterpenes.Antimicrobial activities of the extractsTable 2 shows the zones of inhibitions (mm) of plant extracts at different concentrations (mg/ml). P. aeruginosa showed the highest susceptibility of 14±2mm at 500mg/ml, followed by S. aureus (12±2mm), K. ozanae 10±2mm and E. coli 8±2mm. At concentration of 50mg/ml, P. aeruginosa had the highest zone of inhibition (10±2mm), followed by S. aureus (8±2mm). The least was E. coli with zone of inhibition of 5±1mm. At concentration of 5.0mg/ml, both P. aeruginosa and K. ozanae had the same zone of inhibition of 8±2mm followed by S. aureus (5±1mm) and the least zone of inhibition of 4±1mm was recorded in E. coli.
|
|
|
|
4. Discussion
- The photochemical screening of A. indica extract indicated the presence of saponin and phlobatanins (Table 1), the presence of these compounds may be responsible for the antibacterial activities of the extracts of A. indica on the test organisms. The phytochemical components of the A. indica have been established in previous studies and these include tannins, saponins, alkaloids, carbohydrates, phenols, flavonoids, anthraquinones, cardiac glycosides, sterols and resins (De and Ifeoma, 2002; Natarajan et al., 2003; Biswas et al., 2002, El-Mahmood et al,. 2010). Several studies have linked presence of these bioactive compounds in plant materials to antimicrobial activity.The presence of these secondary metabolites in plants, produce some biological activity in man and animals and it is responsible for their use as herbs. These compounds also serve to protect the plant against infection by microorganisms, predation by insects and herbivores, while some give plants their odors and or flavors and some still are responsible for their pigments (El-Mahmood et al,. 2008). In some cases, the activity has been associated with specific compounds or classes of compounds. These active constituents can be used to search for bioactive lead compounds that could be used in the partial synthesis of more useful drugs (Ogbonnia et al., 2008; El-Mahmood et al., 2010).The antibacterial effects of A. indica on the test organisms revealed that P. aeruginosa showed the highest zones of inhibition (mm) followed by S. aureus while E. coli had the least zone of inhibition (mm) at various extract concentrations of 500mg/ml, 50mg/ml and 5mg/ml (Table 2). Compared to commonly applied antibiotic (Tables 4 and Table 5), bark extracts of A. indica showed a higher value of zones of inhibition on the tested organisms. In a similar study hexane and aqueous extract of Azadirachta indica, inhibited Escherichia coli, P. aeruginosa, S. pyogenes and S.aureus (El-Mahmood et al.,2010).The test organisms had the same minimum inhibitory concentration (MIC) value of 5mg/ml and minimum bactericidal concentration (MBC) value of 50mg/ml. This indicated that the bark of A. indica has similar potency on P. aeruginosa, Kl. ozanae, S. aureus and E. coli. This is similar to the findings of the National Library of Medicine at the National Institutes of Health (www.pubmed.com) who reported that in test tubes A. indica has been shown to have significant effects on both gram-positive and gram-negative organisms and other bacteria that cause a wide array of human and animal diseases.
5. Conclusions
- The results of this study suggest that the bark of A. indica can be used as an antibacterial agent against infections caused by P. aeruginosa, K. ozanae, S. aureus and E. coli.
References
| [1] | Akula, C., Akula, A. and Drew, R. (2003). Somatic Embryogenesis in colonial neem. Azadirachta indica. A. juss. J. Microbiol. Res. 3:162-166 |
| [2] | Biswas, K., Ishita C.., Ranajit K.B. and Uday, B. (2002). Biological activities and medicinal properties of Neem (Azadirachta indica). Current Science 82(11): 1336-1345. |
| [3] | De, N. and Ifeoma, E. (2002). Antimicrobial effects of components of the bark extracts of neem (Azadirachta indica A. juss). J. Technol. Dev., 8: 2328. |
| [4] | EL-Mahmood AM, Doughari JH, Ladan N (2008). Antimicrobial screening of stem bark extracts of Vitellaria paradoxa against some enteric pathogenic microorganisms. Afr. J. Pharm. Pharmacol. 2(5): |
| [5] | El-Mahmood, A. M., Ogbonna, O. B. and Raji, M. (2010). The antibacterial activity of Azadarichta indica (neem) seeds extracts against bacterial pathogens associated with eye and ear infections, Journal of Medicinal Plants Research 4(14), pp. 1414-1421, |
| [6] | Hugo, S.B. and Rusell, A.D. (1983). Pharmaceutical Microbiology 3rd Edition. Blackwell Scientific Publication, London pp. 105-125 |
| [7] | Kayode A.A.A. and Kayode, O.T. (2011). Some Medicinal Values of Telfairia occidentalis: A Review. American Journal of Biochemistry and Molecular Biology, 1: 30-38. |
| [8] | Natarajan, V., Veugopal, P.V. and Menon, T. (2003). Effect of Azadirachta indica (neem) on the growth pattern of dermatophytes. Indian J. Med. Microbiol., 21: 98-101. |
| [9] | National Library of Medicine at the National Institutes of Health, available at www.pubmed.com. |
| [10] | Ogbonnia, S.O., Enwuru, N.V., Onyemenen, E.U., Oyedele, G.A. and Enwuru, C.A. (2008). Phytochemical evaluation and antibacterial profile of Treculia Africana Decne bark extract on gastrointestinal bacterial pathogens. Afr. J. Biotechnol., 7(10): 1385-1389. |
| [11] | Shah, J.S., Shah, M.B., Goswami, S.S. and Santani, D.D. (2006). Mechanism of action of antiulcer activity of bark extracts of Manikara hexandra against experimentally induced gastric ulcers in rats. Phcog. Mag., 2: 40-45. |
| [12] | Silver, O.A., Cabrita, T., Pimentel, M., Diniz, A. and Gomes, E. (1997). Antimicrobial activity of Guinea Bissau traditional remedies. Journal of Ethnopharmacology, 50: 55-59. |
| [13] | Subapriya, R. and Nagini, S. (2005). Medicinal properties of neem leaves: a review, Curr Med Chem Anticancer Agents 5(2):149-6. |
| [14] | Talwar, G.P., Raghuvanshi, P., Misra, R., Mukherjee, S. and Shah, S. (1997). Plant immunomodulators for termination of unwanted pregnancy and for contraception and reproductive health, Immunol Cell Biol., 75(2):190-2. |
| [15] | Trease, G.E. and Evans, W.C. (1989). A textbook of pharmagnosy 13th edition. Baluiere, Tindali, London pp. 100-101 |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML