-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Life Sciences
p-ISSN: 2163-1387 e-ISSN: 2163-1395
2012; 2(2): 1-4
doi: 10.5923/j.als.20120202.01
Phytochemical Potential of Annona reticulata Roots for Antiproliferative Activity on Human Cancer Cell Lines
H M Suresh 1, B Shivakumar 2, S I Shivakumar 3
1Department of Pharmacognosy, HKE Society’s, College of Pharmacy, Gulbarga 585 105, India and Department of Biotechnology, Acharya Nagarjuna University, Guntur, Andhra Pradesh, India
2Department of Pharmaceutical Chemistry, BLDEA’s College of Pharmacy, Bijapur 586 103, Karnataka, India
3Department of Pharmacology, HKE Society’s, College of Pharmacy, Gulbarga 585 105, India
Correspondence to: H M Suresh , Department of Pharmacognosy, HKE Society’s, College of Pharmacy, Gulbarga 585 105, India and Department of Biotechnology, Acharya Nagarjuna University, Guntur, Andhra Pradesh, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Phytochemical and pharmacological activities of Annona reticulata components suggest a wide range of clinical application in lieu of cancer therapy. Present study includes investigation of bioactive constituents from roots of Annona reticulata for cytotoxic properties against different cancer cell lines. Three aporphine alkaloids liriodenine (AAR-01), norushinsunine (AAR-02), reticuline (AAR-03) and one acetogenin neoannonin (TAR -01) isolated from the roots of Annona reticulata. The structures of the compounds were achieved by1HNMR, 13CNMR and Mass spectroscopic methods. All the isolated compounds subjected for cytotoxicity evaluation against A-549, K- 562, HeLa, MDA-MB cancer cell lines and normal cell lines (Vero cells) by MTT assay. All the isolated compounds exhibited prominent dose-dependent cytotoxicity against all the cancer cell lines at dilutions 5, 10 and 20 μg/ml, whereas TAR -01 showed strong cytotoxicity against cancer cell lines with IC50 values ranging from5.8 – 6.9µg/ml. Simultaneously, the effect of all the isolated compounds against Vero cell lines was lower in comparison with the cancer cell lines. The better cytotoxicity of all the isolated compounds the appearance of hydroxyl group at C-7 in AAR-02 could be favourable for increased cytotoxicity against cancer cell lines among aporphine alkaloids and the presence of two hydroxyl groups adjacent to the tetrahydrofuran ring in TAR-01 may be responsible for enhanced activity. The lower cytotoxicity against Vero cell line seems that the isolated constituents AAR-01, AAR-02, AAR-03 and TAR -01 may be used as chemopreventive agents in cancer therapy.
Keywords: Annona Reticulata, A-549, K- 562, HeLa, MDA-MB, Vero Cell Lines, MTT Assay
Article Outline
1. Introduction
- Aporphine is one of a class of quinoline alkaloids and acetogenins are polyketides of many carbons formed by chain extension to form tetrahydrofuran and lactone rings. Both are widely distributed among the plants of annonaceae family. Many potent relatives of these compounds have been purified from the plants. They possess various pharmacological activities such as antiplatelet, anti-tumor cytotoxic and antibacterial activities [1, 2]. Though cancer treatment by modern system of medicines using synthetic drugs is better, search for newer natural drugs continues because of some complications like cell injury, bone marrow depression, impair growth, sterility and hair loss associated with synthetic drugs [3]. Annona reticulata linn, commonly called as bullock’s heart or raamphal plant, is widely distributed all over India and are tall, with many branches, bearing nutritious fruits. The leaves are used as insecticides, anthelmintic, styptic and are also used externally as suppurant. The bark as a powerful astringent is used as antidysentric and vermifuge. Root bark, leaves and stem possess isoquinoline alkaloids [4]. In our early report, we investigated the in vitro antiproliferative activity of ethanol extract of roots againstA-549, k- 562, HeLa and MDA-MB human cancer cell lines [5]. In continuation of our research work on evaluation of bioactive constituents from the roots of this plant for cytotoxic properties, three aporphine alkaloids liriodenine (AAR-01), norushinsunine (AAR-02) and reticuline(AAR -03)and one acetogenins (TAR-01) were isolated and evaluated for cytotoxicity against A-549, K-562, HeLa, MDA-MB and Vero cell lines by performing MTT [3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyl tetrazolium bromide] colorimetric assay.
2. Materials and Methods
2.1. Plant Material
- The roots of Annona reticulata were collected from local areas of north Karnataka and identified by Dr. Srinath Rao of Botany department, Gulbarga University, Gulbarga. A botanically authenticated voucher specimen (HGUG No. 5007) has been deposited at the botany department herbarium of the Gulbarga University, Gulbarga, India.
2.2. Material and Reagent
- MTT assay kit was purchased from Roche Applied Sciences, Germany. A-549 (Human lung carcinoma) , K-562 (Human chronic Myelogenous Leukemia Bone Marrow), HeLa (Human Cervix) and MDA- MB (Human Adenocarcinoma Mammary Gland) and Vero (African green monkey kidney Normal cell) cell lines, free from any bacterial and fungal contamination were procured from NCCS , Pune. All the chemicals and reagents viz Propanol ( Qualigens), Fetal Bovine Serum (Bioclot) and MTT dye were used for the study.
2.3. Isolation of Aporphine Alkaloids
- Air dried and coarsely pulverized roots (2 kg) of Annona reticulata extracted with ethanol (95%). The obtained extract was concentrated to dryness in a rota evaporator at room temperature to obtain ethanol extract (600gm). About 50 gm of concentrated ethanol extract with 2× 250 ml petroleum ether (40 -60°) to separate the fats. The complete defatted marc obtained after extraction was dried and made alkaline with 2× 250 ml ammonium hydroxide (NH4OH) and filtered. The alkaline filtrate further fractionated with 4× 250 ml of chloroform. The organic fractions were pooled and together made alkaline with ammonium hydroxide (NH4OH) washed with water, dried and the solvents removed to yield gummy residue (20 gm). It was partitioned with dichloromethane to remove the neutral components. This residue showed alkaloidal composition on TLC and further used for isolation of alkaloids by column chromatography (neutral alumina) using solvent system toluene: ethyl acetate: diethyl amine (70:20:10). Elution carried out gave a light brown colored substance. After washed with methanol, it was recrystallised from MeOH: acetone (1:1) to give (AAR- 01) pale yellow mass (101 mg).Elution further carried with chloroform: diethylamine (9:1) resulted with another single alkaloidal compound. After removing solvent from the mixed fraction, a residue resulted as a mixture of alkaloid compound which was pale mass (477 mg) followed by re-chromatography on alumina bed using solvent system petroleum ether- chloroform (7: 3) afforded two pure alkaloid compounds designated as AAR- 02 pale brown amorphous powder (116 mg) and AAR -03yellow amorphous mass (99 mg).
2.4. Isolation of Acetogenin
- About 50 gm of ethanol extract partitioned with ethyl acetate subjected for column chromatography [F-254, mesh 60] using solvent system with increasing order of polarities of n: hexane, ethyl acetate and methanol. This resulted with acetogenin mixture followed by re-chromatography with n: hexane: acetone (3:1) solvent system yielded a single acetogeninTAR-01 compound (210 mg) based on TLC confirmation (benzene: acetone; 8:2).Liriodenine (AAR-01) Pale yellow powder (C2H5OH), MS m/z: 274 [M + H] +. 1HNMR (400 MHz, CDCl3): δ 9.14 (1H,s, NH), 9.24 – 7.0 (6H, m, aromatic protons), 6.8 (2H, d, O-CH2-O). 13CNMR (100 MHz, CDCl3) δ 179.22 (C-6), 179.127, (C-5), 165.618 (C-2), 164.506 (C-1), 135.9 (C-13), 135.5 (C-12), 135.5 (C-15), 132.4 (C-9), 129.2 (C-14), 129.17 (C-7), 129.1(C-10), 125.6 (C-8), 124.5 (C-4), 119.6 (C-16), 118.3 (C-17), 104.3 (C-3), 101.2 (C-11). Norushinsunine (AAR-02) Light brown solid mass (C2H5OH), MS m/z: 280 [M + H] +. 1HNMR (400 MHz, CDCl3): δ 6.6 (2H, d, OCH2), 4.0 - 7.9 (11H, m, aromatic protons), 3.9 (1H, s, C-OH), 1.7 (1H, s, NH).13CNMR (100 MHz, CDCl3) δ 139.5 (C-2), 138.5 (C-1), 137.8 (C-14),129.0 (C-15), 128.9 (C-9), 125.8(C-8),123.3 (C-12), 120.2 (C-10), 118.30 (C-17),115.0 (C-7), 111.0 (C-16), 100.0 (C-3),78.07 (C-11), 70.9 (C-6), 62.8 (C-13), 43.3 (C-5), 31.2 (C-4).Reticuline (AAR-03) Yellow powder (C2H5OH), MS m/z: 329 [M + H] + 1HNMR (400 MHz, CDCl3): δ 6.6- 8.3 (6H, m, aromatic protons), 5.7(2H, d, OH), 3.2 (6H, m, OCH2), 1.2-2.8 (9H, m, protons of rings CH2 and side chain CH2). 13CNMR (100 MHz, CDCl3) δ161.3 (C-10), 160.30 (C-9), 158.4 (C-3), 150.7 (C-2), 149.2 (C-15), 129 (C-16), 119.8 (C-13), 117.4 (C-12), 103.7 (C-8), 89.6 (C-1), 79.6 (C-11), 79.3 (C-14), 59.2 (C-17 & C-18), 58.2 (C-6), 52.13 (C-19), 51.0 (C-7), 42.6 ( C-4), 41.3 (C-5).Neoannonin (TAR-01) Pale waxy solid residue (C2H5OH), MS m/z: 578 [M + H] +,1HNMR ( 400 MHz, CDCl3): δ 7.2 (1H,s,C= CH), 4.76 ( 1H, m,CH-CH3 at H34), 4.73 (2H, m , 2CH2 protons, H-17, H-18 ), 3.7 – 3.68 ( 2H, m, 2CH2 protons, H-14, H-21), 3.63 ( 2H,m,CH-OH protons, H-13,H-22), 2.62 - 0.8 (46H, m,23xCH2 protons), 1.9 – 1.6 ( 4H, m, methylene protons, H-15, 16,19,20) , 1.3 ( 3H, m, O-C-CH3). 13CNMR (100 MHz, CDCl3) δ178.1 (C-1), 155.8 (C-33), 130.15 (C- 2), 78.1(C- 17), 78.2(C-18), 77.3 (C-14), 77.3(C- 21), 76.5(C-13), 76.5(C-22), 76.3(C-34), 39.6(C- 26), 39.5(C-30), 37.9(C-20), 37.9(C-15), 33.0(C- 12), 31.8(C-23), 31.4(C- 25), 29.7(C-5), 29.64(C- 6), 29.60(C-7), 29.4(C- 8), 29.2(C-9), 29.1(C- 10), 29.0(C-16),29.0(C-19), 28.67( C-31), 27.3(C-27), 27.3(C- 28), 27.1(C- 29), 25.5(C- 4), 25.3(C-31), 24.2(C- 3), 22.6(C- 11), 18.37(C- 24), 14.0( C- 32).
2.5. Evaluation of Cytotoxicity by MTT Assay
- Both ethanol and aqueous extracts were evaluated for in vitro cytotoxicity study on MDA- MB - 435 (Human melanoma cells) and Vero (African green monkey kidney Normal cell) cell lines by employing MTT assay [6]. The monolayer cell culture was trypsinized and the cell count was adjusted to 3-lakhcells/ ml using medium containing 10% newborn calf serum. To each well of 96 well micro titre plates, 0.1ml of diluted cell suspension of different cell lines was added separately. After 24 hours, when the monolayer formed the supernatant was flicked off and 100μl of AAR-01, AAR-02 and AAR-3 each at the concentration 5, 10 and 20μg in buffered DMSO were added to the cells in micro titre plates separately and kept for incubation at 37℃ in 5% CO2 incubator for 72 hour and cells were periodically checked for granularity, shrinkage, swelling. After 72 hour, the sample dilution in wells was flicked off and 50μl of MTT dye was added to each well. The plates were gently shaken and incubated for 4 hours at 37℃ in 5% CO2 incubator. The supernatant was removed, 50 μl of Propanol was added, and the plates were gently shaken to solubilize the formed formazan. The absorbance was measured using a micro plate reader at a wavelength of 490 nm [7]. Graph of absorbance against concentration of test substance was plotted and inhibitory effect (IC50) was calculated as the drug concentration that is required to reduce absorbance to half that of the control, based on dose - response curve for different isolated substances. The reduction of MTT can only occur in metabolically active cells, the level of activity is a measure of viability of cells. Absorbance values that are lower than the control cell lines reveals decline in the rate of cell proliferation. Conversely, a higher absorbance indicates an increase in the cell proliferation. Untreated micro titre plates of cell lines with only vehicle (0.3 % v/v DMSO in water) is considered as proliferative control.The percent inhibition of cell proliferation by the isolated compounds was calculated based on formula [100–(Mean OD of individual test substance/ Mean OD of control group)] ×100.
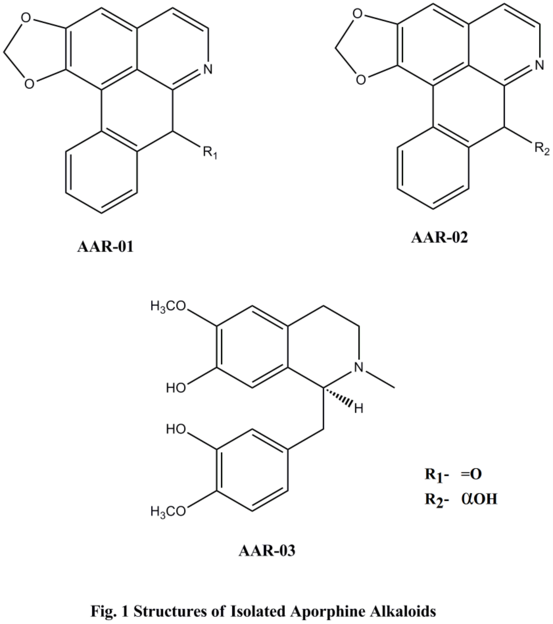 | Figure 1. Structures of Isolated Aporphine Alkaloids |
3. Results
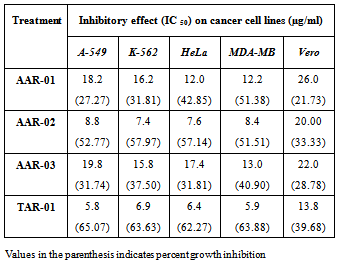
- In the present study three aporhine alkaloids, liriodenine (AAR-01), norushinsunine (AAR-02), reticuline (AAR-03) and one acetogenin, neoannonin (TAR-01) were isolated from the crude ethanol extract of Annona reticulata. The structures of aporhine alkaloids and acetogenin were elucidated by spectroscopic methods, including 1HNMR, 13CNMR and Mass spectra and depicted in Figure 1and 2 respectively. However, they were identified by analysis of their spectral data and by comparison with those previously reported in the literature[8,9].All the isolated compounds (AAR-01, AAR-02, AAR-03 and TAR -01)exhibited dose – dependent cytotoxicity against different cancer cell lines at dilutions 5, 10 and20 µg/ml. Mean cytotoxicity (IC50) and percent inhibition of cell growth results of the isolated compounds against human cancer cell lines (A-549, K-562, HeLa, MDA- MB ) and normal cells ( Vero cell lines) are shown in the [Table 1]. Among the isolated compounds, neoannonin (TAR-01) exhibited strongest cytotoxicity with IC50 values ranging from 5.8 to 6.9 µg/ml against A-549, K-562, HeLa and MDA- MB cancer cell lines. Interestingly, among the aporphine alkaloids, norushinsunine (AAR-02) showed better cytotoxicity with IC50 values ranging from 7.4 to 8.8 µg/ml. Cytotoxicity of test compounds on Vero cell line was constantly less at experimented dilutions as compared with cancer cell lines with IC50 values ranging from 13.8 to 26.0 µg/ml.
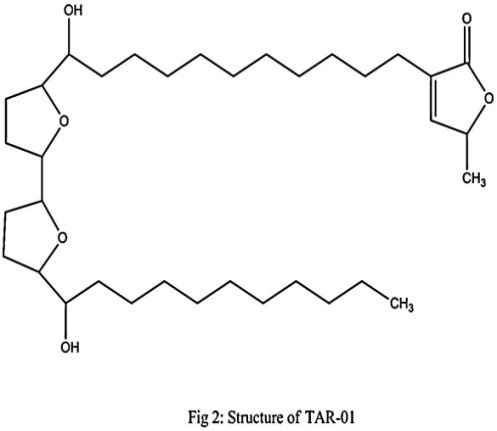 | Figure 2. Structure of TAR-01 |
|
4. Discussion
- Compound AAR-01( Liriodenine); light yellow powder with molecular formula C17H9NO3, compound AAR-02 (Norushinsunine); light brown solid mass with molecular formula C17H15NO3, compound AAR-03( Reticuline); yellow powder with molecular formula C19H23NO4 and compound TAR-01 (Neoannonin); Pale waxy solid residue with molecular formula C35H62O6, as determined from their molecular ion peaks at m/z 274 [M + H] +, 280[M + H] +, 329 [M + H] + and 578 [M + H]+ respectively. The structures of the isolated compounds based on spectral data conclusively revealed that they were liriodenine (AAR-01), norushinsunine (AAR-02), reticuline (AAR-03) and neoannonin (TAR-01). Aporphine and acetogenin compounds are mostly discovered from annonaceae family[10].Among the isolated compounds, liriodenine (AAR-01) is oxoaporphine of 7- substituted aporphines, norushinsunine (AAR-02) is 7- hydroxyaporphine and reticuline (AAR-03) is benzyltetrahydroisoquinoline[11,12]. Further, neoannonin (TAR-01) is dihydroxybistetrahydrofuran fatty acid lactone. Prominent cytotoxicity results of the isolated aporphine alkaloids may be because of isoquinoline moiety in their structures. More pronounced cytotoxicity of AAR -02 among aporhine alkaloids against all the cancer cell lines suggests that appearance of hydroxyl group at C-7 with cis- configuration may be responsible. Remarkably, the strongest cytotoxicity of TAR-01compared to other compounds may be because of presence of two hydroxyl groups adjacent to tetra hydro furan ring. The interesting point we have considered is that all the isolated compounds exhibited weaker cytotoxicity against normal cell lines (Vero cells). The isolated compounds- mediated cytotoxicity was more confined to the cancer cell lines rather than to the normal cell lines. This indicates that the specific cytotoxicity may be due to apoptosis inducing ability of isolated compounds in response to defective gene expression in cancer cell lines rather than the normal cell line because apoptosis is physiologically programmed process of active cellular self destruction responsive to gene expression [13].
5. Conclusions
- In summary, the results of the present study provide convincing evidence that the aporphine alkaloids and acetogenins present in the roots of Annona reticulata may be responsible compounds for cytotoxicity potential against cancer cell lines. The prominent cytotoxic compounds TAR-01 (neoannonin) and AAR-02 (norushinsunine) can be used as a prototype for the development of new synthetic/ semi-synthetic analogues for cancer treatment. However, the less prominent cytotoxic effect of isolated compounds on Vero cell line seems that these compounds may be used as chemopreventive agents in cancer therapy but the precise mechanism by which they exerts this effect needs further investigations.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML