-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Life Sciences
p-ISSN: 2163-1387 e-ISSN: 2163-1395
2012; 2(1): 31-37
doi: 10.5923/j.als.20120201.05
Optimization of Laccase Production from Penicillium martensii NRC 345
A. M. Elshafei 1, M. M. Hassan 1, B. M. Haroun 2, M. A. Elsayed 1, A. M. Othman 1
1Department of Microbial Chemistry, National Research Centre, Dokki, Cairo, Egypt
2Department of Botany and Microbiology, Faculty of Sci., Al-Azhar University, Cairo, Egypt
Correspondence to: A. M. Elshafei , Department of Microbial Chemistry, National Research Centre, Dokki, Cairo, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Laccase production by Penicillium martensii NRC 345 was optimized. Eight media with different components were screened. The enzyme formed by P. martensii NRC 345 was detected mainly exocellularly under shake culture condition. Laccase formation reaches its maximum value with specific activities of about 7.18 U/mg protein at the twenty-sixth day of incubation at pH 5.5 and 30°C. Galactose (5 g/l) and sodium nitrate (0.2 g/l) were found to be the best carbon and nitrogen sources for laccase formation, respectively. Replacement of galactose instead of glucose at the same concentration increased laccase production by about more than ten-fold. Among the various wastes used wheat bran induces the highest laccase production with specific activity of 39.52 U/mg protein. Tween-80 and CuSO4.5H2O, were also tested for laccase induction.
Keywords: Laccase, Penicillium martensii, production, optimization
Cite this paper: A. M. Elshafei , M. M. Hassan , B. M. Haroun , M. A. Elsayed , A. M. Othman , "Optimization of Laccase Production from Penicillium martensii NRC 345", Advances in Life Sciences, Vol. 2 No. 1, 2012, pp. 31-37. doi: 10.5923/j.als.20120201.05.
Article Outline
1. Introduction
- Laccases (benzenediol: oxygen oxidoreductase, EC1.10.3.2) are either mono or multimeric copper- containing oxidases that catalyze the one-electron oxidation of large numbers of phenolic substrates. Molecular oxygen serves as the terminal electron acceptor and consequently reduced to two molecules of water[1]. Laccase catalyses the four electron reduction of oxygen to water coupled with single-electron oxidation of four hydrogen-donating substrates[2]. This is accomplished by a cluster of four copper atoms (type 1 copper; type 2 copper and two type 3 copper atoms) that form the enzyme active site[3]. Type 1 copper, which confers the typical blue colour to the enzyme due to intense electronic absorption of covalent copper–cysteine bonds, is the site where monoelectronic oxidation of substrate takes place. Type 2 and type 3 copper atoms form a trinuclear cluster, to which the electrons are transferred and where molecular oxygen is reduced to water[4]. Laccases are widely distributed in nature, originating from plants, insects, bacteria and fungi[5]. In fungi, laccases are widely distributed in ascomycetes, deutromycetes and basidiomycetes. These laccase producing fungi include Trametes (Coriolus) versicolor, T.villosa, T. hirsute, T. gallica, T. ochracea, Cerrena maxima, Phlebia radiata, Lentinus tigrinus, Coriolopsis polyzona and Pleurotus eryngii. Laccases are also reported in saprophytic ascomycetes of com- posts (Myceliophthora thermophila, Curvularia, Penicillium, Aspergillus and Chaetomium thermophile) and in the soil hyphomycete Mycelia sterlia INBI 2-26[6, 7].Many laccase applications were reported some of which have been patented, ranging from effluent decoloration and detoxification to pulp bleaching, removal of phenolics from wines and dye transfer blocking functions in detergents, washing powders and degradation of lignin[1].The optimization of medium to reduce the cost of laccase is the basic research in the industrial applications[8]. Another approach is to overproduce laccase in a suitable host. Thus, screening studies should be made to select new promising microorganisms producing laccase[9]. The purpose of this work is to study the cultural and nutritional conditions for laccase production by a filamentous fungus which was not previously described in the literature.
2. Materials and Methods
2.1. Microorganism
- Penicillium martensii NRC 345 was obtained from Culture Collection of Microbial Chemistry Department, National Research Centre, Egypt.
2.2. Chemicals and Media
- Syringaldazine was supplied by Sigma, USA. The other chemicals used in this study were of analytical grade and higher purity. A culture was maintained through periodic transfer on slants of potato-dextrose agar (PDA) medium, and was kept at 4℃. The liquid medium used for laccase production by the fungal culture during its growth in submerged fermentation was composed as follows; 5g of glucose, 1g of KH2PO4, 0.5g of MgSO4.7H2O, 0.2g of NaNO3, 0.1g of yeast extract, 0.01g of CaCl2, 1mg of CuSO4.5H2O, 1mg of FeSO4.7H2O, 1mg of MnSO4.7H2O and 1000 ml of distilled water[10]. The liquid medium was then adjusted to pH value 5.0, and sterilized by autoclaving at 1.5 atmospheres and 121℃ for 20 min.
2.3. Preparation of Cell Free Filtrates
- At the end of the incubation period, the cultures were filtered using Whatman No.1 filter paper. The culture filtrate was used directly as a crude enzyme preparation.
2.4. Preparation of Cell Free Extracts
- P. martensii NRC 345 pellets were filtered and homogenized in citrate phosphate buffer (100 mM; pH 5.0) and cold washed sand in a cold mortar (4℃). The crude homogenate was then centrifuged at 5000 rpm for 10 min. The upper layer containing cell free extracts was separated by decantation and used as the crude endocellular enzyme preparation[11].
2.5. Enzyme Assay
- Laccase activity was assayed by monitoring the product formation rate of enzymatic oxidation of syringaldazine spectrophotometrically at 525nm (ε525 = 65000 M−1 cm−1)[12]. The assay mixture (2.0 ml) contained syringaldazine, 0.1µmole; citrate-phosphate buffer (pH 5.0), 90 µmoles and appropriate volume of diluted enzyme. One unit of laccase activity was defined as the change in the absorbance of 0.001 per sec[13]. Protein content was estimated by the modified procedure of Lowry et al.,[14]. All the data was statistically evaluated according to the method described by Kenney and Keeping[15]. The means and standard deviations of means (Mean ± S.D.) were calculated for each experiment.
3. Results
3.1. Effect of Media Composition
- P. martensii NRC 345 was grown on eight media described by different investigators under both static and shake culture conditions (120 rpm) using New Brunswick scientific Co. Inc. Edison N. J. USA shaker at 28 ± 2℃ for 26 days of incubation and the cell-free filtrate (CFF) and cell-free extract (CFF) were then used as enzyme sources. The eight liquid media were tested for inducing laccase production with their different compositions. Results indicate that, P. martensii NRC 345 laccase was mainly formed under shake condition. Results also showed that in the diverse composition of the culture media used for the cultivation of P. martensii NRC 345, better enzyme production was obtained maximally in medium No 1 (7.76 U/mg protein) under shake culture condition followed by medium No 2. No laccase activity could be detected in case of using media number 4, 5 and 6 under shake culture condition and media number 3 and 5 under static condition (Table 1). In all successive experiments, medium No 1 under shake condition will be used.
3.2. Localization of Laccase
- P. martensii NRC 345 was grown on medium No 1 under static and shake culture conditions. Laccase activity levels in both cell-free filtrate (CFF) and cell-free extract (CFE) were determined.Results obtained showed that, the enzyme formed by P. martensii NRC 345 expressed as specific activities, was detected mainly in the exocellular fraction (CFF) under shake culture condition. Relative specific activities of the corresponding endocellular enzyme values only represent about 22 % and 16 % with static and shake conditions respectively, which indicate that, the enzyme is mainly excreted exocellulary (Table 2).
| ||||||||||||||||||||||
3.3. Effect of Incubation Period
- P. martensii NRC 345 was grown on media No 1 under shake culture condition (the best tested media for laccase formation), at pH 5.0 for thirty-one days of incubation. Samples of the fermented medium were collected periodically for the determination of laccase activity. Results indicate that the highest specific activity was obtained at the twenty-sixth day of incubation with specific activity of 7.18 U/mg protein. After this incubation period, a gradual decrease in enzyme formation level was occurred (Figure 1).
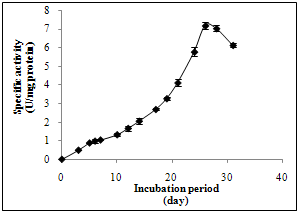 | Figure 1. Effect of incubation period on laccase formation by P. martensii NRC 345 |
3.4. Influence of Initial pH Values
- The effect of pH on laccase production was carried out by incubating the culture flasks (250 ml Erlenmeyer conical flasks) each containing 50 ml of production medium at different pH values. A series of pH values ranging from 4.0 to 7.0 were studied. From the obtained results, it is clear that maximal formation of P. martensii NRC 345 laccase took place at pH 5.5, and laccase formation occurred at a narrow range of pH values, whereas considerably low levels of enzyme were obtained at pH values below and higher than this value (Figure 2).
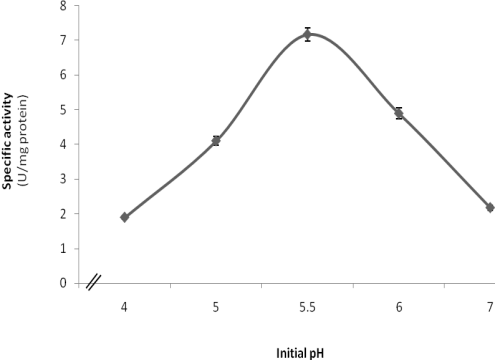 | Figure 2. Influence of different initial pH values on the formation of P. martensii NRC 345 laccase |
3.5. Effect of Incubation Temperature
- The present experiment aims to determine the optimal incubation temperature for laccase formation by P. martensii NRC 345, which was grown at various degrees of temperatures ranging from 25 to 35℃. Results demonstrated that the optimal temperature for laccase formation was found to be 30℃ with specific activity of 7.21 U/mg protein, and below or above this incubation temperature value a considerable decrease in laccase formation was detected (Figure 3).
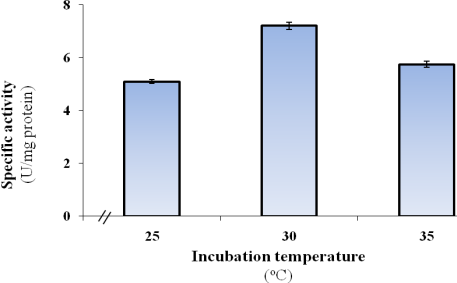 | Figure 3. Influence of incubation temperature on the formation of P. martensii NRC 345 laccase |
3.6. Effect of Inoculums' Size
- The present experiment is an attempt to find out the optimal inoculum size for laccase production. Therefore, inocula with size ratios ranging from two to seven agar plugs (14 mm in diameter) were cut from actively growing fungal mycelia and inoculated in the production medium. Results indicated that, as the percentage of the introduced inoculum size was raised from two to five discs, laccase progressively increased. The optimal inoculum size was found to be five discs. Further increase in inoculum size reduced the level of enzyme formation (Figure 4). Consequently, five discs inoculum size, which was the optimal for laccase production from P. martensii NRC 345, was used in the subsequent experiments.
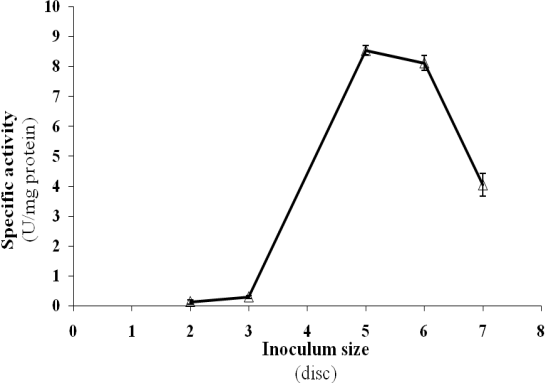 | Figure 4. Influence of inoculums' size on the formation of P. martensii NRC 345 laccase |
3.7. Influence of Lignocellulosic Wastes on Laccase Formation
- Different lignocellulosic wastes (wheat bran, wheat straw, saw dust, rice bran, rice straw, corn stalk, corn stover and sugar cane bagasse) were used instead of glucose in the medium for laccase production by P. martensii NRC 345. Results cited in Table (3) showed that among the various wastes used, wheat bran followed by corn stover were found to be best substrates for laccase production by P. martensii NRC 345 with maximum laccase specific activity (39.52 and 33.33 U/mg protein, respectively). On the other hand, the lowest activity was obtained with wheat straw when used as a substrate. No detectable laccase activity was found when saw dust was used as a substrate instead of glucose.
|
3.8. Effect of Different Carbon Sources
- This experiment aims to study the effect of different carbon sources on the formation of laccase by P. martensii NRC 345. Each carbon source was added at a concentration of 0.5 % to the medium described by[10] (the best media for laccase formation), as the main only carbon source.As shown in Figure (5), galactose was found to be the best carbon source for laccase formation by P. martensii NRC 345. Alternatively, CMC completely repressed laccase production. On the other hand, all tested carbon sources stimulated laccase production than glucose (the carbon source in the original medium). Galactose reduced the incubation period to be 22 days only instead of 26 days in case of glucose in the original medium. It is worthy to mention that, replacement of galactose in the medium described by[10] instead of glucose at the same concentration increased laccase production by about ten-fold.
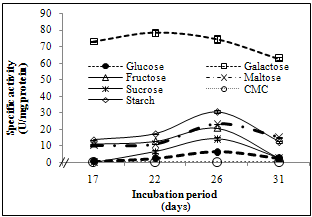 | Figure 5. Influence of different carbon sources on the formation of P. martensii NRC 345 laccase |
3.9. Formation of Laccase as a Function of Galactose Concentration
- From the preceding experiment, it has been detected that galactose was the best carbon source for laccase production by P. martensii NRC 345. Therefore, in this experiment the effect of galactose concentration on enzyme formation was tested. Galactose was added to the culture medium instead of glucose at five different concentrations ranging from 2.5 to 20 g/l. It is clear from Figure 6, that the enzyme specific activity was increased with increasing galactose concentration up to 5 g/l, which is the best concentration of galactose for laccase formation. By increasing galactose concentration above this value, a gradual decrease in laccase activity occurred.
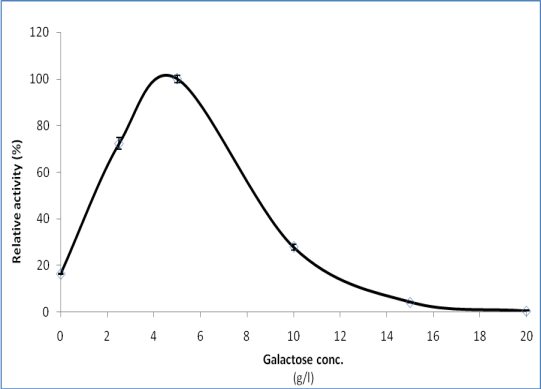 | Figure 6. Influence of galactose concentration on the formation of P. martensii NRC 345 laccase |
3.10. Effect of Nitrogen Sources
- Various nitrogen compounds were added separately to the culture medium in amounts equivalent (on nitrogen basis) to the amount of nitrogen in sodium nitrate (0.2 g/l) in the modified medium described by[10] using galactose as a carbon source instead of glucose. Table 4 demonstrated that, the highest level of enzyme formation expressed in terms of specific activity was obtained with sodium nitrate (76.56 U/mg protein) followed by L (+) glutamine (53.92 U/mg protein). On the other hand, no enzyme formation could be detected with ammonium sulfate or ammonium chloride.
|
3.11. Influence of Addition of Tween-80 and Copper Sulfate
- The effect of addition of Tween-80 and copper sulfate at concentrations between 0.1 – 0.75 % (v/v) and 50 - 1500μM respectively, indicated that both supplements had no induction effect on laccase production by P. martensii NRC 345 (data not shown).
4. Discussion
- The objective of this article was to enhance laccase production by P. martensii NRC 345, which was not previously described in the literature. The medium components, carbon source, nitrogen source, initial pH and incubation temperatures were studied to investigate their effects on laccase production. Previous studies indicated that these factors were considered to be important for laccase production by white rot fungi[22]. Eight liquid media with different compositions were tested for inducing laccase production by Penicillium martensii NRC 345. Results indicated that, within the diverse composition of the culture media used for the cultivation of P. martensii NRC 345, better enzyme production (7.76 U/mg protein) was obtained maximally in the medium described by Chawachart et al.[10] in the exocellular fraction (CFF) under shake culture condition and this culture medium was found to enhance laccase formation than other tested culture media. The present research has demonstrated that incubation period is a determining factor in the process of laccase production by P. martensii NRC 345 because the production reaches its maximum value in a relatively gradual manner and then falls. The production decline after attaining the maximum might be due to the depletion of macro-and micronutrients in the fermentation medium with the lapse in time, which stressed the fungal physiology resulting in the inactivation of secretary machinery of the enzymes[23]. Zeng et al.[24] stated that, the production of ligninolytic enzymes by most white-rot fungi was found to be a ‘secondary metabolic’ event. Concerning the delay of optimum laccase formation which reached the 26th day of incubation in our study Fortina et al.[25] reported that, Botrytis cinerea produced appreciable levels of laccase (9.8 U/ml) in a brief period (5-7 days) and they stated that, some fungal species required a longer production time (12-30 days).Most of fungal cultures prefer a slightly acidic pH in the medium for growth and enzyme biosynthesis[26] in agreement with the results obtained. In this study, maximal formation of P. martensii NRC 345 laccase took place at pH 5.5 with a narrow range of pH values. This may be attributed to the fact that change in pH value may alter the three-dimensional structure of the enzymes[27]. In the present study, the optimum production of laccase by this fungus occurred at a temperature of 30℃ (7.21 U/mg protein), with a specific activity of 5.10 U/mg protein at 25℃. In this context, Zadrazil et al.[28] reported that, temperatures higher than 30°C caused reduction in ligninolytic enzymes activity. In our study, different lignocellulosic wastes (wheat bran, wheat straw, sawdust, rice bran, rice straw, corn stalk, corn stover and sugar cane bagasse) were used instead of glucose in the medium for laccase production. Srinivasan et al.[29] stated that, the cellulose and lignocellulosic residues were considered to stimulate laccase production. The presence of water-soluble constituents from lignocellulose fractions of the lignocellulosic residues such as p-coumaryl alcohol, coniferyl alcohol, sinapyl alcohol and other aromatic compounds might result in the increased laccase activities in comparison to the growth on glucose[30]. Results in this study showed that, among the various wastes used, wheat bran followed by corn stover were found to be best substrates for laccase production by P. martensii NRC 345 with maximum laccase specific activity (39.52 and 33.33 U/mg protein, respectively) when used separately as substrates instead of glucose. In order to improve laccase production, a wide range of carbon sources added separately in the medium was examined. The production of enzymes is related to the type and concentration of the carbon source in the medium[31], and according to[32], a substance that presents itself as an enzymatic inducer for one species can be an inhibitor of the same activity in another species. Andrade et al.[33] reported that, glucose is one of the most used carbon sources for biomass growth because it is easily metabolized but according to our results, galactose was found to be the best carbon source for laccase formation by P. martensii NRC 345 which reduced the incubation period to 22 days only instead of 26 days in case of glucose in the original medium. The replacement of galactose instead of glucose at the same concentration increased laccase production by about more than ten-fold. Dhawan and Kuhad[34] reported that both nature and the concentration of nitrogen source are of considerable importance in laccase production. Results also demonstrated that, the highest level of laccase formation by P. martensii NRC 345 expressed in terms of specific activity was obtained with sodium nitrate (78.56 U/mg protein) followed by L (+) glutamine (53.92 U/mg protein). Zeng et al.[35] and Palmieri et al.[36] reported to the stimulatory effect of surfactants and copper sulfate on laccase production. Opposite to this facts, in this study, P. martensii NRC 345 grew on the medium supplemented separately with different concentrations of Tween-80 and copper sulfate ranging from 0.1 to 0.75 % (v/v) and from 50 to 1500μM respectively, showed no obvious promotion of laccase activity, which indicated that the inducing capability of these compounds might vary among different lignin-degrading fungi.
5. Conclusions
- In view of the results obtained, it can be concluded that: media composition, culture conditions, pH values, incubation temperatures, inoculums' size, carbon sources and nitrogen sources have significant influence on laccase production. The optimization of these criteria leads to overproduction of laccase by P. martensii NRC 345. It is worthy to mention that, replacement of galactose instead of glucose increased laccase production by about more than ten-fold. However, such results point out the necessity of a better understanding of production process, mainly regarding to induction and repression processes of the substrates used.
ACKNOWLEDGEMENTS
- The authors acknowledge the support of the National Research Center, Cairo, Egypt; which enabled authors to accomplish this work. Authors also would like to acknowledge the late-Prof. Dr. Osama Mohamed Abdel Fattah; Division of Genetic Engineering and Biotechnology, National Research Centre, Cairo, Egypt, for his technical support, sound advices and sincere help throughout this work.
References
| [1] | Poonkuzhali, K., Sathishkumar, P., Boopathy, R. and Palvannan, T. 2011. Aqueous state laccase thermostabilization using carbohydrate polymers: Effect on toxicity assessment of azo dye. Carbohydrate Polymers, 85: 341–348. |
| [2] | Baldrian, P. 2006. Fungal laccases—occurrence and properties. FEMS Microbiol. Rev., 30: 215–42. |
| [3] | Riva, S. 2006. Laccases: Blue enzymes for green chemistry. Trends Biotechnol., 24 (5): 219–26. |
| [4] | Kudanga, T., Nyanhongob, G. S., Guebitzb, G. M. and Burton S. 2011. Potential applications of laccase-mediated coupling and grafting reactions: A review. Enzyme and Microbial Technology 48: 195–208. |
| [5] | Poonkuzhali, K. and Palvannan, T. 2011. Thermostabilization of laccase by polysaccharide additives: Enhancement using central composite design of RSM. Carbohydrate Polymers, 86: 860– 864. |
| [6] | Vasil’chenko, L.G., Koroleva, O.V. Stepanova, E.V. Landesman, E.O. and Rabinovich, M.L. 2000. Isolation and characteristics of micromyetes-producers of neutral phenol oxidase from tropic soil with a high level of dioxins. Prikl. Biokhim. Mikrobiol., 36: 412–421. |
| [7] | Dwivedi, U. N., Singh, P., Pandey, V. P. and Kumar, A. 2011. Structure–function relationship among bacterial, fungal and plant laccases: A review. Journal of Molecular Catalysis B: Enzymatic 68: 117–128. |
| [8] | Pointing, S. B., Jones, E. B. G. and Vrijmoed, L. L. P. 2000. Optimization of laccase production by Pycnoporus sanguineus in submerged liquid culture. Mycologia, 92: 139–44. |
| [9] | Li, P., Wang, H., Liu, G., Li, X. and Yao, J. 2011. The effect of carbon source succession on laccase activity in the co-culture process of Ganoderma lucidum and a yeast. Enzyme and Microbial Technology, 48: 1–6. |
| [10] | Chawachart, N., Khanongnuch, C., Watanabe, T. and Lumyong, S. 2004. Rice bran as an efficient substrate for laccase production from thermotolerant basidiomycete Coriolus versicolor strain RC3. Fungal Diversity, 15: 23-32. |
| [11] | Verdin, A., Sahraoui, A. L. H. and Durand, R. 2004. Degradation of benzo[a]pyrene by mitosporic fungi and extracellular oxidative enzymes. International Biodeterioration & Biodegradation 53: 65 – 70. |
| [12] | Minussi, R. C., Miranda, M. A., Silva, J. A., Ferreira, C. V., Aoyama, H., Marangoni, S., Rotilio, D., Pastore, G. M. and Durán, N. 2007. Purification, characterization and application of laccase from Trametes versicolor for colour and phenolic removal of olive mill wastewater in the presence of 1-hydroxybenzotriazole. African Journal of Biotechnology, 6 (10): 1248-1254. |
| [13] | Aríca, M. Y., Altıntas, B. and Bayramoğlu, G. 2009. Immobilization of laccase onto spacer-arm attached non-porous poly (GMA/EGDMA) beads: Application for textile dye degradation. Bioresource Technology, 100: 665–669. |
| [14] | Lowry, O. H., Rosebrough, N. J., Farr, A. L. and Randall, R. J. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem., 193: 265- 275. |
| [15] | Kenney, J.F. and E.S. Keeping, 1962. The standard deviation and calculation of the standard deviation" In Mathematics of statistics, Pt1,3rd ed. Princeton, NJ, Van Nostrand, pp. 77-80. |
| [16] | Chefetz, B., Chen, Y. and Hadar, Y. 1998. Purification and Characterization of laccase from Chaetomium thermophilium and its role in humification. Appl. Environ. Microbiol., 64 (9): 3175–3179. |
| [17] | Da Re, V., Papinutti, L., Villalba, L., Forchiassin, F. and Levin, L. 2008. Preliminary studies on the biobleaching of loblolly pine Kraft pulp with Trametes trogii crude extracts. Enzyme and Microbial Technology, 43: 164–168. |
| [18] | Dhaliwal, R. P. S., Garcha, H. S. and Khanna, P. K. 1992. High laccase producing Pleurotus florida mutants. World Journal of Microbiology and Biotechnology, 8: 39-41. |
| [19] | Bora, B. 2003. Production of laccase by the phytopathogenic fungus Rhizoctonia solani. Ph D. Murdoch University, Perth, Western Australia. |
| [20] | Tlecuitl-Beristain, S., Sánchez, C., Loera, O., Robson- Geoffrey, D. and Díaz-Godínez, G. 2008. Laccases of Pleurotus ostreatus observed at different phases of its growth in submerged fermentation: Production of a novel laccase isoform. Mycological Research, 112:1080-1084. |
| [21] | Khlifi, R., Belbahri, L., Woodward, S., Ellouz, M., Dhouib, A., Sayadi, S. and Mechichi, T. 2010. Decolourization and detoxification of textile industry wastewater by the laccase-mediator system. Journal of Hazardous Materials, 175: 802–808. |
| [22] | Jaouani, A., Tabka, M. G., Sayadi, S., Vanthournhout, M. and Penninck, M. J. 2004. Potent fungi for olive oil mill wastewaters decolourisation. International Biodeterioration and Biodegradation, 53: 224. |
| [23] | Simões, M. L. G., Tauk-Tornisielo, S. M. and Tapia, D. M. T. 2009. Screening of culture condition for xylanase production by filamentous fungi. African Journal of Biotechnology, 8 (22): 6317-6326. |
| [24] | Zeng, G. M., Yu, H. Y., Huang, H. L., Huang, D. L., Chen, Y. N., Huang, G. H. and Li, J. B. 2006. Laccase activities of a soil fungus Penicillium simplicissimum in relation to lignin degradation. World Journal of Microbiology and Biotechnology, 22: 317–324. |
| [25] | Fortina, M.G., Acquti, A., Rossi, P., Manachini, P. L. and Gennaro, C. Di. 1996. Production of laccase by Botrytis cinerea and fermentation studies with strain F226. Journal of Industrial Microbiology, 17: 69-72 |
| [26] | Haltrich, D., Nidetzky, B., Kulbe, K. D., Steiner, W. and Zupancic, S. 1996. Production of fungal xylanases. Biores. Technol., 58: 137- 161. |
| [27] | Shulter, M. L. and Kargi, F. 2000. Bioprocess engineering basic concept, Prentice Hall of India Pvt Ltd, New Delhi, India. |
| [28] | Zadrazil, F., Gonser, A. and Lang, E. 1999. Influence of incubation temperature on the secretion of extracellular ligninolytic enzymes of Pleurotus sp. and Dichomitus squalens into soil. Proceedings of the Conference on Enzymes in the Environment: Activity, Ecology and Applicants. 12-16 July. Granada, Spain. |
| [29] | Srinivasan, C., Dsouza, T. M., Boominathan, K. and Reddy, C. A. 1995. Demonstration of laccase in the white-rot basidiomycete Phanerochaete chrysosporium Bkm-F1767. Applied and Environmental Microbiology, 61: 4274-4277. |
| [30] | Schlosser, D., Grey, R. and Fritsche, W. 1997. Patterns of ligninolytic enzymes in Trametes versicolor distribution of extra- and intracellular enzyme activities during cultivation on glucose, wheat straw and beech wood. Applied Microbiology and Biotechnology, 47: 412-418. |
| [31] | Gawande, P. V., and Kamat, M. Y. 2000. Production of xylanases by immobilized Aspergillus sp. using lignocellulosic waste. World J. Microb. Biot., 16: 111-112. |
| [32] | Hrmova, M., Beily, P. and Vrsanka, M. 1989. Cellulose and xylan degrading enzymes by Aspergillus terreus. Enzyme Microb. Tech., 11: 610-616. |
| [33] | Andrade, V. S., Sarubbo, L. A. and Fukushima, K. 2002. Production of extracellular proteases by Mucor circinelloides using D- glucose as carbon source / substrate. Braz. J. Microbiol., 33(2): 106-110. |
| [34] | Dhawan, S. and Kuhad, R. C. 2002. Effect of amino acids and vitamins on laccase production by the bird’s nest fungus Cyathus bulleri. Bioresource Technology 84: 35–38. |
| [35] | Zeng, G. M., Shi, J. G., Yuan, X. Z., Liu, J., Zhang, Z. B., Huang, G. H., Li, J. B., Xi, B. D. and Liu, H. L. 2006. Effects of Tween 80 and rhamnolipid on the extracellular enzymes of Penicillium simplicissimum isolated from compost. Enzyme Microb. Technol., 39: 1451- 1456. |
| [36] | Palmieri, G., Giardina, P., Bianco, C., Fontanella, B. and Sannia, G. 2000. Copper Induction of Laccase Isoenzymes in the Ligninolytic Fungus Pleurotus ostreatus. Appl. Environ. Microbiol., 66 (3): 920–924. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML