-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Life Sciences
p-ISSN: 2163-1387 e-ISSN: 2163-1395
2012; 2(1): 6-19
doi:10.5923/j.als.20120201.02
Remedial Use of Withanolides from Withania Coagolans (Stocks) Dunal
Maryam Khodaei1, Mehrana Jafari2, Mitra Noori2
1Dept of Chemistry, University Of Sistan & Baluchestan, Zahedan Post Code: 98135-674 Islamic republic of Iran
2Dept. of Biology, University of Arak, Arak, Post Code: 38156-8-8349, Islamic republic of Iran
Correspondence to: Mehrana Jafari, Dept. of Biology, University of Arak, Arak, Post Code: 38156-8-8349, Islamic republic of Iran.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Withanolides are a branch of alkaloids, which reported many remedial uses. Withanolides mainly exist in 58 species of solanaceous plants which belong to 22 generous. In this review, the phyochemistry, structure and synthesis of withanolieds are described. Withania coagulans Dunal belonging to the family Solanaceae is a small bush which is widely spread in south Asia. In this paper the biological activities of withanolieds from Withania coagulans described. Anti-inflammatory effect, anti cancer and alzheimer’s disease and their mechanisms, antihyperglycaemic, hypercholesterolemic, antifungal, antibacterial, cardiovascular effects and another activity are defined. This review described 76 compounds and structures of Withania coagulans.
Keywords: Withanolides, Withania Coagulans, Solanaceae, Biological Activity
Cite this paper: Maryam Khodaei, Mehrana Jafari, Mitra Noori, Remedial Use of Withanolides from Withania Coagolans (Stocks) Dunal, Advances in Life Sciences, Vol. 2 No. 1, 2012, pp. 6-19. doi: 10.5923/j.als.20120201.02.
Article Outline
1. Introduction
- Withania coagulans Dunal is very well known for its ethnopharmacological activities (Kirthikar and Basu 1933). The W. coagulans, is common in Iran, Pakistan, Afghanistan and East India, also used in folk medicine. Fruits of the plant have a milk-coagulating characteristic (Atal and Sethi 1963). The fruits have been used for milk coagulation which is attributed to the enzymatic charisma of the plant (Naz et al 2009). The fruits are sweet and are reported to be sedative, emetic, alterative and diuretic. They are useful in chronic disorders of liver. The fruits are also used in dyspepsia, flatulent coli and other intestinal infections. They are employed for treatment of asthma, biliousness and strangury. In some parts of the Indian sub-continent, the berries are used as a blood purifier. The twigs are chewed for cleaning teeth and the smoke of the plant is inhaled for relief of toothache (Kirthikar and Basu 1933). Chadha (1976) said Withania coagulans is also known as a treatment of ulcers, rheumatism, bronchitis, and degenerative diseases. (Refer to Atta-ur- Rahman 1998 -e)
2.1. Taxonomical Classification
- Genus: Withania, Family: Solonaceae, Subfamily: Solanoideae,Tribe: Physaleae, Subtribe: Withaninae,Species: Withania coagulans (Stocks) Dunal. (Hemalatha et al. 2008)
2.2. Distribution
- Drier parts of Punjab, Gujarat, Simla and Kumaon in India, Baluchestan in Iran, Pakistan and Afghanistan.
2.3. Synonyms
- English: Vegetable Rennet, Indian-Cheese-maker, Unani- Desi Asgandh, Kaaknaj-e-Hindi, Paneer, Paneer-band, Akri (fruit), Siddha/Tamil-Ammukkura.Local names: This plant is known by different names, in different local languages, such as 'Akri' or 'Puni-ke-bij' in Hindi, 'Tukhme-kaknaje-hidi' in Persian. 'Spiubajja' in Afghan, 'Khamjira' in Punjabi and 'Punirband' or 'Punir- ja –fota' in Sindhi (Naz 2002).
2.4. Botanical Description
- W. coagulans is a rigid gray-whitish small shrub, 30-90 cm tall, leaves 2.5-7.5 cm by 1.5 cm, usually lanceolate oblong, sometimes ovate, obtuse, entire, and narrowed at the base and very short stalked. They are densely covered with minute, gray, stellated tomentum. Flowers are 7-12 mm across, yellow dioeciously and polygamous. They are in axillary cymose clusters, leathery calyx; seeds are dark brown, ear shaped, glabrous, pulp brown, having sharp fruity smell (Dymock et al 1893).
2.5. Useable Part
- Whole plant, roots, leaves, stem, green berries, fruits, seeds and bark are used.Fruits: Carminative, depurative, used for dyspepsia, flatulence and strange. The properties are attributed to the pulp and husk of the berry. The berries contain a milk- coagulating enzyme, esterase, free amino acids, fatty oil, an essential oil and alkaloids (Khare 2007). The milk- coagulating activity is due to the presence of an enzyme, under optimum conditions (Atal and Sethi 1963). Ashwagandha (Bengali) and Ashwagandhi (Kannada) are confusing synonyms of W. coagulans (Khare 2007).Seeds: anti-inflammatory, emetic, diuretic, emmenagogue.Leaf: alterative, febrifuge.
3. Withanolides
- The term “withanolide” is a structural term that has been used for “withan” from the genus Withania, and “olide” is chemical term for a lactone. To this date, about 400 withanolides or closely related congeners have been discovered in altogether 58 solanaceous species belonging to 22 genera (Eich 2008). Withanolides have been discovered also in certain Tacca spp. of the Taccaceae (taccalonolides) (Huang et al 2002) and Ajuga spp., e.g., A. parviflora Benth. Lamiaceae (ajugins) (Khan et al 1999), as well as in some marine organisms. Nevertheless, their occurrence in the Solanaceae is predominating by far (Eich 2008). Different withanolides, withacoagin and coagulan reported from W. coagulans. Withaferin A (Steroidal lactones of withanolide series) had been isolated from fruits of W. coagulans (Khare 2007).
3.1. Structures
- Withanolides of ergostan steroids are four-ring triterpenes. The plant steroids are derived from sterols and comprise steroid saponins, steroid alkaloids, pregnanes, androstanes, estranes, ecdysteroids, withanolides and cardiac glycosides (Kreis and Muller-Uri 2010). “Withanolide” represents the term for the C28-skeleton 22-hydroxyergostan-26-oic acid -22, 26-olide; this γ-lactones residue containing the structure is a theoretical (Lavie et al 1965 a, b). The basic skeleton of withanolides is shown in Figure 1.Basically there are two major groups of withanolides as follows: A- Withanolides with an unmodified skeleton a) With a regular β -oriented side chain b) With an unusual α -oriented side chain. B- Withanolides with modified carbocyclic skeletons or side chains. These withanolides are initially classified on the basis of the chemotypes of the Withania species depending on the region of the collected plant. Chemically, these compounds may be classified as ergostane derivatives from their structural pattern; these can be broadly divided into seven groups.1. 5β, 6 β -epoxides 2. 6 α, 7 α -epoxides3. 5-enes4. Intermediate compounds5. 5 α, 6 α -epoxides 6. 6 β, 7 β -epoxides 7. Phenolic withanolides (Glotter 1991).Among these, the 5β, 6β -epoxides are most common. Most of the compounds possess a 4β -hydroxy1 group. Purushothaman and Vasanth (1989) extracted four-ring compound which possess α, β -unsaturated-γ-lactone system, in e.g. ixocarpa- lactone A. (Refer into Atta-ur-Rahman 1998e). Ray (1989) showed that the withanolides which possess 6α and 7α-epoxides generally contain 5α -hydroxyl and are believed to originate from 5β, 6β -epoxides (Refer to Atta-ur- Rahman 1998e).
3.2. Synthesis
- Withanolides generally contain a polyoxygenated ergostan skeleton. One of the characteristics is the ability to introduce oxygen functions in almost every position of the carrbocyclic skeleton and side chain of compounds of this type (Naz 2002). Withanoloieds are synthesized via the mevalonate pathway of terpenoids formation and arise from the initial cyclization of 3S-squalene-2, 3-epoxide (Kreis and Muller-Uri 2010).
3.3. Phytochemistry
- Different phytochemistry studies have been done on W. coagulans and various compounds have been isolated from the plant. The phytochemical investigations on W. coagulans up to 2011 reported a number of phytoconstituents. The most important constitutions of W. coagulans are shown in Table 1 and their structures are shown in Figure 2.
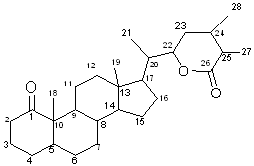 | Figure 1. Basic skeleton of withanolides |
4. Remedial Uses
4.1. Anti-Inflammatory Effect
- The alcoholic extract of W. coagulans showed significant anti-inflammatory effects in acute inflammation induced with egg albumin. Sub-acute inflammation induced with formalin and granulation tissues were formed by cotton pellet method (Budhiraja et al 1977). Budhiraja et al (1986) reported Anti-inflammatory activity of a withanolide from W. coagulans.
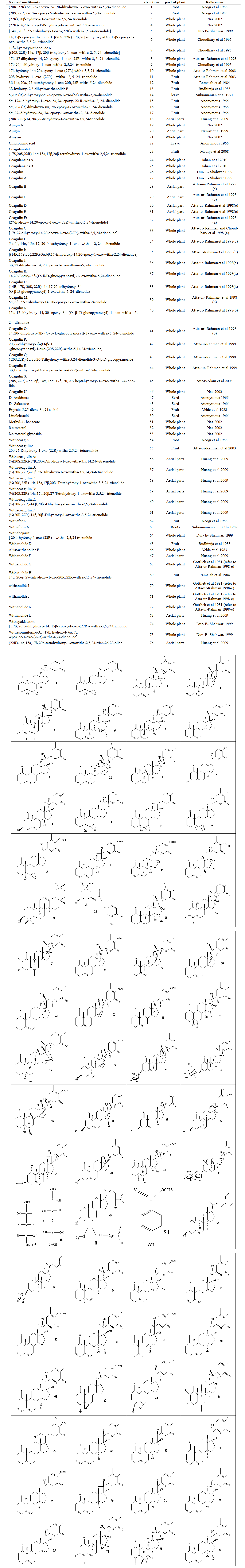 | Figure 2. Structures of withanoleides for more details refer to Table 1 |
4.2. Anti Cancer
- Withacoagulin A, withacoagulin C, withacoagulin D, withacoagulin E, withanolide L, , withanolide J, Δ3 iso withanolide F, withanolide F, withacoagulin and (22R)- 14α15 α17β- 20 β, tetrahydroxy-1oxowitha 2,5,24 trien, 26,22-olide compounds had relatively good activities (IC50<20 mm) on the inhibition of both Con A-induced T cell and LPS-induced B-cell proliferation that, among these compounds, withanolide F had the strongest activity (IC50¼1.66 mm) and the best SI value (25.5). Also withacoagulin C exhibited a satisfactory SI value. Withanolides induces apoptosis in HL-60 leukemia cells via mitochondria then the cytochrome C is released and caspase activation (Senthil et al 2007). 3F-hydroxy-2, 3dihydro- withanolide F was reported to possess anti-tumor activity (Budhiraja et al 1987). John et al (1998) reported that the extract of W. coagulans has the potential to inhibit thymidine incorporation and antiproliferative activity. The aqueous extract of W. coagulans was studied for its anti-cytotoxic effect. The extract showed remarkable DMSO (Dimethyl sulfoxide) inhibitory activity which was induced to produce cytotoxicity and decreased the TNF-G production in chicken Lymphocyte (Chattopadhyay et al 2007). Withanolide A is well-known for its neuronal regenerating effect; it would be also dangerous to simply imply that this compound could be an excellent anti-dementia drug. It would be first necessary to investigate the side effects of the bioactive compounds and their possible interactions, to develop more clinical experiments (Mirjalili et al 2009). It is well established that the various compounds of Withania species, such as withaferin A from the leaves, are known to posses anti-cancer properties (Jayaprakasam et al 2003). They have been reported to inhibit the cell growth of various human cancer cell lines, including lung cancer (NCI-H460). Withaferin A showed antiproliferative activity against head and neck squamous carcinoma, by reduced cell viability in cell lines in vitro (Subramanian et al 1969). This mechanism of action is a part of the result of G2/M cell cycle arrest and induction of apoptosis in HNSCC cells. Withaferin A inhibited the activation of Akt and reduced total Akt levels. Additional studies will be required to evaluate the potential of withaferin A activity application, for future anticancer drug development in head and neck squamous carcinoma (Samadi et al 2010).Phytochemical studies on withaferin A has shown cytotoxity in vitro, against KB cell cultures derived of intramuscular carcinoma (Fluka et al 1987). The studies also showed growth inhibitory and radio sensitizing effects in vivo on mouse Ehrlich ascites carcinoma (a transplantable, poorly differentiated malignant tumor as a spontaneous breast carcinoma in a mouse) (Devi et al 1995). Withaferin A showed significant anticancer activity in animals cell cultures by decreasing the expression of the nuclear factor-kappa β and suppressing the intercellular tumor necrosis factor, therefore has the potential of apoptotic signaling in cancerous cell lines (Ichikawa et al 2006). Withaferin A showed inhibition growth and cytotoxic activity against human lung cancer cell lines (NCI-H460) (Choudhary et al 2010). The mechanism by which withanolides demonstrates antiproliferative, antimetastatic, antiangiogenic, anti-invasive, and proapoptotic activities was associated with the suppression of NF-jB and NF-jB-regulated gene products (Ichikawa et al 2006). The precise role of these substances in tumor promotion is not clear, but a correlation of elevated prostaglandins in carcinogenesis has been established (Lupulescu 1978) and certain nonsteroidal anti-inflammatory agents (e.g. indomethacin and piroxicam) demonstrate chemopreventive activity assessed by Reddy et al (1992) (Atta-ur-Rahman 1998-e). It has evaluated that the plant extracts have potential to inhibit cyclooxygenase (Verma et al 1980, Jang and Pezzuto 1997). One of the most promising agents which have been identified as an inhibitor of cyclooxygenase is resveratrol. In addition to the blocking of the cyclooxygenase activity of the enzyme, the hydroperoxidase activity is inhibited. A related effect is the inhibition of COX-2 (this enzyme induced during the process of tumor promotion). So research for specific inhibitors of COX-2, versus COX-1 and a more favorable profile of activity would be anticipated (Jang et al 1997). The mechanism of action of COX-1 in treatment of rheumatism might be attributed to the inhibition of T- and B-cell functions (Huang et al 2009).
4.3. Alzheimer’s disease
- Pathologically, Alzheimer’s disease is characterized by extracellular deposits of amyloid beta (Aβ) protein and intracellular accumulation of neurofibrillary tangles (NFTs) that are composed of hyperphosphorylated tau (τ) protein (Mattson 2004; Selkoe 2001). Familial Alzheimer’s has been proven to be associated with mutations in AβPP genes (Chartier-Harlin et al 1991; Sherrington et al 1995; Levy- Lahad et al 1995). Withanolide A has been studied for its potential activities against multiple associated targets with Aβ pathways (BACE1, ADAM10, IDE, and NEP). BACE1 (beta-site APP cleaving enzyme 1) is a rate-limiting enzyme in the production of Aβ from amyloid- precursor protein (AβPP), while ADAM10 (Disintegrin and metalloproteinase domain- containing protein 10) is involved in non- amyloidogenic processing of AβPP. IDE and NEP are two of the prominent involved enzymes in effective degrading Aβ. It was found out that withanolide A significantly down- regulates BACE1 and also up-regulates ADAM10 in primary rat cortical neurons. These compounds may be proven to be valuable in developing novel, effective therapeutics for the prevention and treatment of associated Alzheimer’s disease with amyloid pathology (Patil et al 2010). W. coagulans has been studied for possible spasmolytic and calcium channel blocking effects. Potassium chloride (KCl) induced contractions also the W. coagulans crude extract possesses calcium channel blocking activity (Ali et al 2009). Spasmolytic and Ca2+ antagonistic potentials in isolated rabbit, 5, 20 α (R)-dihydroxy-6α, 7α-epoxy-1-oxo-(5α)-witha-2, 24- dienolide is active on spontaneous and K+ induced contractions. The cholinesterase inhibitory potential along with calcium antagonistic ability and safe profile in human neutrophil viability could make this withanolide possibly a candidate for further study to treat Alzheimer’s disease and its associated problems (Choudhary et al 2005).
4.4. Antihyperglycaemic
- The drug W. coagulans exhibited hypoglycaemic activity which is an effective and safe alternative treatment for diabetes (Gurson and Saner 1971; Budhiraja et al 1977; Hemalatha et al 2004). Isolated alkaloids and steroids from plant sources are responsible for hypoglycemic activity of those sources (Adebajo et al 2006). Significant improvements in symptoms and signs were observed and euglycemia was attained (diabetes mellitus type 2) by Lopez-Ridaura et al (2004) and Jaiswal et al (2009). Also the extracted coagulin L from W. coagulans fruits has antihyperglycemic activity in rats (Maurya et al 2008). Lalsare and Chutervedi (2010) reported that various extracts of W. coagulance fruits to have anthihyperlipidemic activity. The aqueous and chloroform extracts of the fruits decreased the blood glucose (55%), also the fruits aqueous extract decrease blood glucose by (52%), (Hoda et al 2010). Extracted coagulin L from fruits of W. coagulans was determined about 25 mg/kg in streptozotocin-induced diabetic rats, which is comparable to the standard antidiabetic drug metformin (Maurya et al 2008).The main minerals play a contributory role in enhancing hypoglycemic activity (Kar and Choudhary 1994; Kar et al 1999). Decreasing of blood glucose level and the improving of glucose tolerance test significantly showed that the higher content of Mg and Ca in W. coagulans is responsible for their significant role in diabetes management (Kumar et al 2009; Rai et al 2007; Giugliano et al 2000). It has been already reported that higher concentrations of Mg and lower concentrations of K play a vital roles in diabetes management (Lopez-Ridaura et al 2004, Fox et al 2001). Hence the significant antidiabetic potential of W. coagulans could be due to the high concentration of Mg along with Ca. The Ca2+ ion activates insulin gene expression via CREB (Calcium Responsive Element Binding Protein) and is responsible for exocytose of stored insulin (Veiga et al 2006).
4. 5. Hypercholesterolemic
- The aqueous extract of W. coagulans fruits in high fat diet induced hyperlipidemic rats, significantly reduced elevated serum cholesterol, triglycerides, lipoprotein and the LPO levels. This drug also showed hypolipidemic activity in induced triton hypercholesterolemia. The hypolipidemic effect of W. coagulans fruits were found to be comparable with ayurvedic product containing Commiphora mukkul (Hemalatha et al 2006). The extracted coagulin L from fruits of W. coagulans has antidyslipidemic effect on mice (Maurya et al 2008). Hoda et al (2010) showed the aqueous and chloroform extracts of the fruits decreased triglyceride, total cholesterol, LDL and VLDL increased the HDL levels.
4.6. Antifungal and Antibacterial Effects
- The volatile oil from the fruits of W. coagulans showed antibacterial activity against Staphylococcus aureus and Vibrio cholerae (Choudhary et al 1995, Khan et al 1993). Also antibacterial properties have been demonstrated for isolated withanolides from ethanolic extract of the leaves (Gaind and Budhiraja 1967). Two withanolides (14,15β -epoxywithanolide I [(20S,22R) 17β,20β-dihydroxy -14β, 15β-epoxy-1-oxo-witha-3,5,24-trienolide] and 17β- hydroxywithanolide K (20S,22R) 14α,17β,20β-trihydroxy- 1-oxo-witha-2,5,24-trien-olide]) have been isolated from the whole plant of W. coagulans. The second compound was found to be active against a number of potentially pathogenic fungi (Choudhary et al 1995). The antifungal activity of the crude extract, 17β-hydroxy withanoloied k and withanolide F were tested against nine highly pathogenic isolated fungi i.e. Nigrospora oryzae, Aspergillus niger, Curvularia lanata, Pleuretus ostreatus, Stachybotrys atra, Allescheria boydii, Drechslera rostrata, Microsporum canis, and Epidermophyton floccosum. These compounds also showed activity against gram positive (S. aureus) (Atta-ur-Rahman and Choudhary 1998). The essential oil was active against Micrococcus pyogenes var. aureus and Vibro cholerae (Khare 2007). Withanolide D has antifungal cytotoxic activity on thirteen fungi which is responsible for human infectious (five dermatophytes, one nondermatophyte mold, six yeasts, and Pneumocystis carinii) (Roumy et al 2010). Lalsare et al (2010) showed antioxidant and antimicrobial activities of various extracts of W.coagulance fruits.
4.7. Cardiovascular Effects
- The biosynthetic pathway lead from phytosterol precursors to the cardiac glycosides (important compounds in the treatment of cardiac insufficiency in humans) basically deduced from studies using radiolabelled precursors (Kreis and Muller-Uri 2010). An isolated new withanolide with a special chemical structure that was similar to the aglycones of the cardiac glycosides was examined for its cardiovascular effects of W. coagulans fruits. The withanolide caused a moderate drop of blood pressure in dogs (34 +/- 2.1, mm Hg) which was blocked by atropine and not by mepyramine or propranolol. In rabbits Langendorff preparation and ECG studies, produced myocardial depressant effects but in perfused frogs hearts it caused mild positive inotropic and chronotropic effects (Budhiraja et al 1983). Extracted coagulin L from W. coagulans fruits also showed significant drop of a fasting blood glucose profile and improved the glucose tolerance of db/db mice (Maurya et al 2008).
4.8. Immunosuppressive Effects
- Withaferin A and withanolide E were reported to have specific immunosuppressive effects on human B and T lymphocytes as well as on mice thymocytes (Shohat et al 1978). The cell-mediated response is generated by various subpopulations of T-lymphocytes. These cells serve to activate various T effector cells that generate cell-mediated responses. The cytokines secreted by activated Th cells also regulate the proliferation and differentiation of a number of effector cells that play various roles in cell-mediated immune responses. After stimulating with PHA, T-cell progression and proliferation is depended on the cytokine production. It is obvious that the interaction between T-cells and antigens or mitogen initiates a cascade of genes expression process for proteins such as IL-2 and IFN- γ that induces the resting T-cells to enter the cell cycle (G0–1 transition) and contributes in the expression of the high affinity receptors for IL-2 and secretion of IL-2 (Tsai et al 2001). Inhibition of T-cell activation provides a powerful approach for immunosuppressive treatment (Mukaida et al 1994).A known withanolide, coagulin-H, was evaluated for its effect on various cellular functions related to immune responses including lymphocyte proliferation, interleukin-2 (IL-2) cytokine expression. These results were compared with prednisolone. Coagulin-H was found to have a powerful inhibitory effect on lymphocyte proliferation and the Th-1 cytokine production. The inhibition of the phytohaemagglutinin (PHA) activated T-cell proliferation by coagulin-H (Mesaik et al 2006).
5. Another Activity
- i. Furthermore, W. coagulans has wound healing activities in streptozotocin-induced diabetic rats. The hydroalcoholic fraction of W. coagulans in oral form is found to be more effective than the aerial part of Aloe barbadensis Miller which is used as a local demulcent in wounded diabetic rats. Prasad et al (2010) showed that withaferin-A is responsible for increasing the collagen significant levels, protein, DNA, SOD, CAT and hexosamine decreasing.ii. As Siddiqui et al (1963) studies showed the extract of Withania coagulans is muscular relaxing in experimental animals and is also a hypotensive, respiratory stimulant.iii. The protective effect of obtained 3F-hydroxy-2, 3 dihydro-withanolide F from W. coagulans fruits was studied against the hepatotoxicity, induced by CCl4. Budhiraja et al (1984) showed that the protective effect of withanolide F was more active than hydrocortisone.iv. The aqueous extract exhibited free radical scavenging activity in an in vitro system using DPPH (Budhiraja et al 1986). Fruits extracts of W. coagulans have antioxidant potential against several diseases such as ageing, artherosclerosis etc. which caused due to ROS. ( Mathur et al. 2011)v. Based on Karami et al (2006) the root extract of W. coagulans had significant effects on the withdrawal syndrome in mice. It also showed significant suppression of morphine withdrawal jump, induced by naloxone, and decreased the development of morphine dependence.vi. Using the aqueous extract of W. coagulans fruits in experimental rats have a diuretic potential. Withanolides from W. coagulans are more polar in nature compared to the other Withania species. The diuretic effects may be associated with the presence of the active principles of polar nature where withanolides are the main chemical protagonist of this activity. Dabheliya et al (2010) investigation's supports using W. coagulans as the diuretic agent in traditional folklore medicine. vii. A steam volatile oil of the petroleum ether extract of W. coagulans has been found to possess lethal effect on earthworms (Gaind and Budhiraja 1967). The aerial parts of W. coagulans have anthelmintics in ruminants (Jabbar et al 2006). Also Khare reported an anthelmintic activity for W. coagulans (Khare et al 2007).
6. Conclusions
- The fruit, leaves and root of Withania coagulans have been used as a treatment in many disorders. It's found to be rich source of esterases, free amino acids, fatty oils, essential oils and withanolides. Withanolides are steroidal lactones and several of them possess significant pharmacological activities. Hence, further structure–activity relationship investigations and possible optimization of their non-toxic and diffusion properties would be interesting. In addition, future studies should lead to synthesis of these complex and fascinating chemical structures and their generics via modification/addition of different functional groups. It is also important to reveal the bio-efficacy of isolated compounds in combination with other herbs or drugs. Moreover, it is also necessary to study the effects and mechanisms of the isolated molecules in vivo using suitable higher animals to ensure its potentiality and safety. The present review comprehensively enlists the isolated compounds. It also describes the remedial qualities and the molecular mechanism of action.
ACKNOWLEDGEMENTS
- The authors are very grateful to dr J. walisade who recommended this plant for research and to dr H.R. shaterian, dr N. kazemipour and dr M. Kaykhaii in recognition of their favourable help in the early stages of the research of this article.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML