-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Life Sciences
p-ISSN: 2163-1387 e-ISSN: 2163-1395
2011; 1(1): 11-19
doi: 10.5923/j.als.20110101.03
Tale of Fish Sperm and Factors Affecting Sperm Motility: A Review
M. Sadiqul Islam , T. Akhter
Department of Fisheries Biology & Genetics, Bangladesh Agricultural University, Mymensingh, 2202, Bangladesh
Correspondence to: M. Sadiqul Islam , Department of Fisheries Biology & Genetics, Bangladesh Agricultural University, Mymensingh, 2202, Bangladesh.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Motility is an important function of the male gamete, which allows sperm to actively reach and penetrate the female gamete in organisms with internal and external fertilization. Sexual activity of some fish is generally seasonal and fertilization is external. Sperm, once differentiated in the gonad, remain there completely quiescent until they are released into the external medium, which is either freshwater or sea water. Various parameters such as ion concentrations (K+, Na+, Ca2+), osmotic pressure, pH, and temperature affect motility. In the present paper, we review the roles of these factors on sperm motility in the teleosts. Studying the effects of these factors on teleost sperm can help establish good activation and/or immobilizing media for improving either artificial fertilization or cryopreservation.
Keywords: Fish, Sperm, Motility, Ion, Osmotic Pressure
Cite this paper: M. Sadiqul Islam , T. Akhter , "Tale of Fish Sperm and Factors Affecting Sperm Motility: A Review", Advances in Life Sciences, Vol. 1 No. 1, 2011, pp. 11-19. doi: 10.5923/j.als.20110101.03.
Article Outline
1. Introduction
- Fish sperm are widely divergent in form and structure[1]. It is not possible to construct a spermatic model for the ‘fishes’, as is the case, for example for the snakes and mammals. They vary from aflagellate to biflagellate and have an enormous range of shapes, sizes, and structures; the number and location of organelles also vary[2, 3, 4]. Both light and electron microscopy of a wide spectrum of teleost sperm have demonstrated that important morphological differences can be found among species[5] and can be used for taxonomic purposes[2]. The structure of the spermatozoon is influenced by both reproductive mode and systematic position[6]. For example, the conclusion of Mattei and Mattei[7] based on the study of sperm of eight species of fishes from the orders Elopiformes and Anguilliformes supported the theory of other workers that these two orders should be grouped together in the super order Elopomorpha. Morphological differences in teleost spermatozoa indicate that submicroscopical morphology of these cells can be useful as additional characters in taxonomic classification as suggested by many researchers[2, 7, 8].Study of the structure and morphology of fish sperm provides information for understanding their possible taxonomic and evolutionary relationships at family[1, 9], subfamily and species[10] levels, as well as for optimizing artificial reproduction, prevention of polyspermy problems and development of cryopreservation techniques[5]. Spermiogenesis and spermatozoon ultra-structure has been studied in a number of teleost fishes with those variations having phylogenetic importance reported[1, 11, 12].Most of the externally fertilizing teleosts cannot swim in the male gonads. Sperm are only become motile and metabolically active after release into the water. Within the gonad, high CO2 tension in semen maintains intracellular pH at ~7.2 with respect to sea water[13]. When sperm are spawned into seawater, the CO2 concentration decreases, H+ release and intracellular pH increases to 7.5-7.6. Dynein, the ATPase that drives the flagella, is inactive below pH 7.3, repressing motility and respiration[14, 15]. In most freshwater species, sperm usually moves for less than 2 min and in many cases is only highly active for less than 30 sec[16, 17, 18, 19]. Some fish species such as the spotted wolfish (Anarhichas minor) and the 3- and the 15-spined sticklebacks (Gasterrosteus aculeatus, and Spinachia spinachia respectively), which are characterized by release of eggs in a sticky gelatinous mass, have sperm which remains motile for a far longer period after release[20, 21, 22]. In a recent finding it was found that Perca fluviatilis (perch) sperm have the ability to swim for more than two hours in saline conditions[23].Sperm motility is the functional parameter that might be influenced directly and most significantly by sperm morphology and structure of sperm. Differences are observed in many species of teleosts in terms of length of the flagellum, number of mitochondria of the sperm which all can affect motility[2, 24]. In some species, the longest sperm swim fastest at high energy costs[25]. In an experiment it was found that perhaps longer sperm had more ATP available for swimming and achieved a higher fertilization success[26] and even sperm with higher velocities of sperm is able tofertilize a greater number of eggs[27]. Earlier it was mentioned that fish sperm remain quiescent in the genital tract and in the seminal plasma and they become transiently motile at spawning when released into the surrounding water. There are several factors that affect sperm motility such as pH, temperature, ions and osmolality[28, 29, 30] which lead to activation of axonemal movement.The aim of the present review is to discuss the structure and morphology of fish sperm and to discuss the effects of physical factors (e.g. temperature) and chemical factors (e.g. pH, ions, osmotic pressure) that affect the motility of fish sperm.
2. Methodology
- An extensive search of PUBMED, ScienceDirect and the author’s files was done without limitations by language or species for citations relevant to fish sperm. Wider searches of citations relevant to morphology, physiology and signal transduction were also performed without limitations of language, species, or date, to broaden the background to this review. All papers that matched the search criteria and were relevant to this review were included. Diagrams were made using power point, collating data from references within the review and the table was generated in excel, by the same means.
3. Morphology and Composition of Fish Sperm
3.1. Anatomy
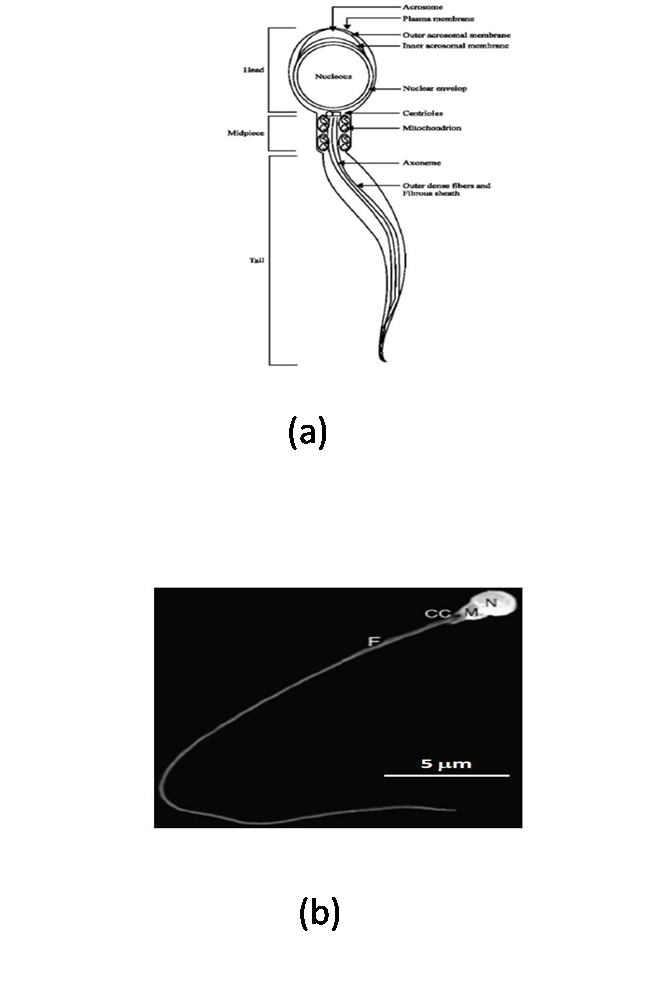
- Sperm are quite small cells and display similar general design in almost all species (Figure 1). It consists of head, mid-piece and flagellum.
3.2. Head
- A head varying in diameter, containing condensed packages of chromosome in the nucleus (which occupies a significant portion of the head) and in some species the acrosome, a membranous structure overlying the nucleus in the anterior part of the sperm head (Figure 1). A proper size and shape of spermatozoon head is a prerequisite for the entering of spermatozoon throughout the micropyle[5]. Generally, the sperm head in fishes is relatively small (2–4 μm) in relation to the total size of sperm. The exceptions were observed at Atlantic eel sperm[5, 31] and at sturgeons and paddlefish Polyodon spathula (Walbaum) with elongated sperm head up to 10 μm in length and over 2 μm in width, containing acrosome[5, 31, 32, 33, 34, 35, 36, 37] or acrosome less[38].Different shapes of sperm head occur in chondrostean and teleostean fishes. For example, in northern pike Esox lucius Lit is regular, ball shaped[39, 40]; big sperm head in silver carp Hypophtalmichthys molitrix V.[41, 42]; ovoid in cardinal fish Apogon imberbis L.[43]; ornate in wrasse Thalassoma pavo L.; kidney-like in damselfish Chromis chromis L.; spherical in Mediterranean rainbow wrasse Coris julis L.[8]; banana-shaped in Atlantic eel Anguilla anguilla L.[44, 45]; highly elongated in Mimagoniates barberi (Regan)[46]; crescent-shaped in Conger myriaster (Berewoort)[47]. In some species (e.g. in perch, Perca fluviatilis L.), the sperm head is laterally flattened[48].
3.3. Mid-piece
- Mid-piece consists of centriole and mitochondria and linked with head (Figure 1). Mitochondria contains at the base of the tail, contributing to power flagellar movement. The midpiece is similar to that of a mammalian sperm, except for the fact that fish sperm contain fewer mitochondria and its flagellum is separated from the midpiece by a cytoplasmic channel[43]. In perch P. fluviatilis, only a single mitochondrion was found in the sperm head[48] while more than 20 mitochondria were found in the midpiece of ide Idus melanotus L.[5] and in cyprinids it varied from 2 to 10[2].In chondrostean and teleostean fishes, only the mitochondrial segment was found recognizable, while centriolar segment was hidden in so-called intranuclear channel[5]. In rainbow trout Oncorhischus mykiss (Walbaum), the distal centriole attached to the transverse axis system includes a free portion nesting on the proximal centriole, which varies in form between a circle and an ellipse. Same size of the both centrioles (30 nm length, 22 nm diameter) are arranged rectangularly to the head base in a roughly cubical depression equivalent to an implantation groove[49].
3.4. Flagellum
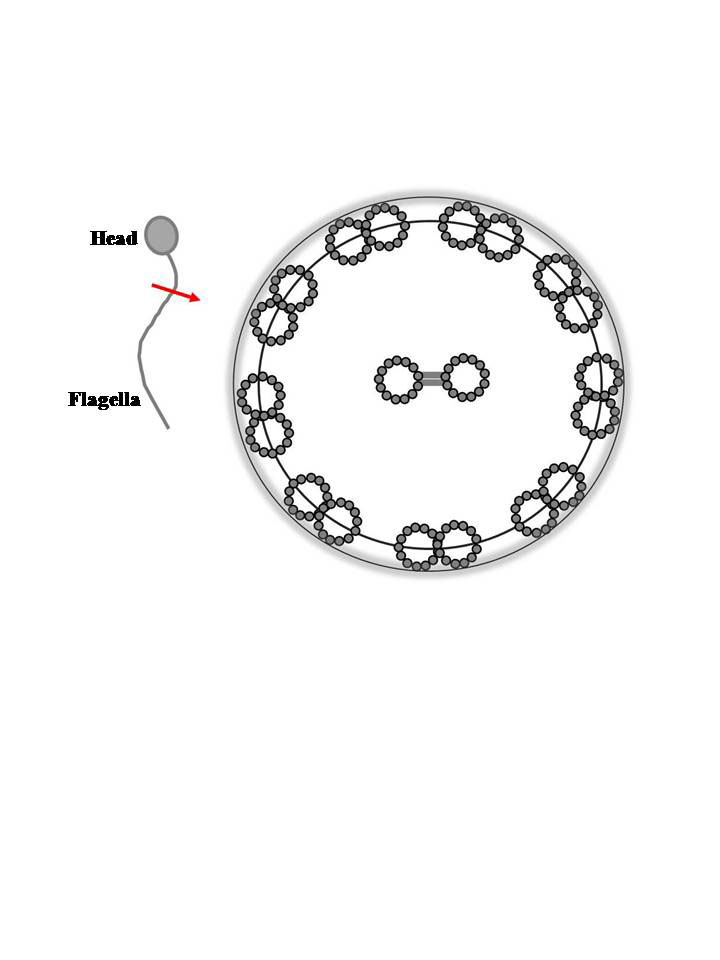
- As the tails of sperm, flagella comprise the motile apparatus necessary to the movement and penetration of sperm into the egg at fertilization. The flagellum which varies in length, depending on the species and contains the axoneme (Figure 1). The length of flagellum in coho salmon (O. kisutch) is about 2.6 μm[50], in channel catfish it is about 94 μm[51] and in C. myriaster it is 37 μm in length[47]. Flagellar length in cyprinid species varies from 36 to 60 μm[2]. The flagellum itself is composed from two central and nine peripheral doublet microtubules, so-called ‘‘9+2 complex’’[41] (Figure 2). The 9+2 structure and molecular composition of the axoneme are well conserved among eukaryotic cilia and flagella from protozoa to human. The nine doublet microtubules are interconnected and the central pair bridge joins the inner microtubules. This characteristic composition, i.e. 9+2 structure with the central microtubules showing identical orientation were present in channel catfish Ictalurus punctatus (Rafinesque), coho salmon Oncorhynchus kisutch (Walbaum), common carp Cyprinus carpio L., loach Misgurnus fossilis L., Siniperca (Siniperca chuatsi, Siniperca kneri, and Siniperca scherzeri)[5, 12, 41, 50, 51] while in Anguilliformes and Elopiformes present a ‘‘9+0’’ structure without central microtubules[7]. Some peculiarities in the flagellum were reported for some species: cells with two flagella were found in plainfin midshipman Porichtis notatus (Girard)[52] and channel catfish I. punctatus[51].
4. Ionic Composition of Fish Milt
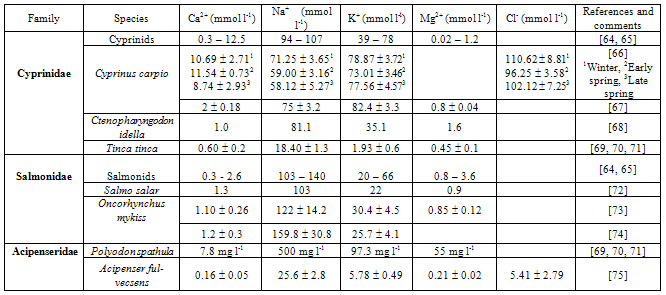
- Milt is defined as sperm plus seminal plasma. Seminal plasma (or fluid) has a unique composition: some components support the sperm, while others reflect the functions of the reproductive system and the sperm[53]. Studies on semen characteristics are necessary to understand the basic biochemical processes that occur in sperm motility and during fertilization[54, 55, 56, 57, 58, 59], to evaluate the reproductive abilities of different fish species[25, 55, 60, 61, 62], and to improve methods for short- and long-term storage of fish milt[63]. The ionic compositions of fish milt in different groups of fishes are summarized in Table 1.
|
5. Sperm Motility Characteristics
- Motility is a characteristic function of the male gamete, which allows sperm to actively reach and penetrate the female gamete in organisms with internal and external fertilization. Sperm motility is acquired under the control of many extrinsic and intrinsic factors and is based on the specialized structure of the sperm flagellum. Generally, sperm of fishes spawning in brackish and sea water swim much longer than those of most freshwater species[76]. However, the duration of sperm motility is brief in most fish species and only lasts for 30 sec to few minutes[77].Sperm of both freshwater and seawater fish are immotile in the male reproductive organ, or in electrolyte or nonelectrolyte solutions having similar osmolality to that of the seminal plasma, but gain potentiality for activation during transfer to the sperm duct. The physiological changes that occur after transfer is controlled by an endocrinological system, which regulate spermatogenesis and spermiation[64, 65, 78]. Seminal plasma produced by the sperm duct provides an ionic environment that maintains the viability of spermatozoa after their release from the testes[79]. It has been already shown in the literature that there are several correlations between seminal plasma composition and sperm motility in some species; Atlantic salmon, Salmo salar[72], common carp, Cyprinus carpio[66], bleak, Alburnus alburnus[74], rainbow trout, Oncorhynchus mykiss[80], Persian sturgeon, Acipenser persicus[81] and chinook salmon, Oncorhynchus tshawytscha[82]. In freshwater species, matured sperm need a hypo-osmotic shock for triggering initiation of sperm motility[29]. Moreover, there are several factors that affect sperm motility such as temperature, pH, ions and osmolality[28, 29, 30, 83].
5.1. Effect of Temperature
- The motility duration, fertilizing ability and velocity of sperm depend on temperature of the activation medium[5, 65, 84] and of that of the broodstock holding tank[85]. Because the energetic resources of fish sperm are limited, an increase in velocity caused by a temperature rise in the swimming solution leads to a shorter duration of motility, and conversely, lowering the swimming temperature results in a prolonged duration of motility and reduced cell velocity[5, 84]. The motility duration of grass carp, Ctenopharyngodon idella sperm is shorter than the common carp[86, 87]. It has been confirmed that sperm are motile for longer at 20℃ than at 26 or 30℃ in common carp or 30℃ in grass carp[87], whereas in Siberian sturgeon, Acipenser baeri, sperm decreases when the temperature is increased from 10 to 17.5℃ [85].
5.2. Effect of pH
- It has been shown that extracellular and intracellular pH, as well as the ionic composition of the activating solution, influences the initiation and duration of sperm motility[88]. The external pH probably influences the intracellular proton concentration, which subsequently affects the membrane potential, as well as motility behavior[89, 90]. In rainbow trout the pH of seminal plasma is usually 7.5 to 8.5. Carp sperm motility can be initiated in media with an external pH of 6.0 - 9.0[91, 92]. On the other hand, the internal pH of the sperm is about 1 unit below the external pH[60, 93].
5.3. Effect of Ions
5.3.1. Potassium (K+)
- Among the above mentioned factors, K+ concentration is a key factor in combination with osmotic pressure that control sperm motility and allow it to be initiated in salmonids[49, 84], sturgeons and paddlefish[53, 62, 67, 69, 70, 71, 81, 94, 95]. It has been known since 1938 that millimolar level of extracellular K ion concentration ([K+]o) in the seminal tract is primarily responsible for keeping trout sperm inactive[96]. This phenomenon was further investigated by some scientists and they showed that salmonid fish sperm motility can be initiated in K+-free medium, but not in K+-supplemented medium, which is similar to the seminal fluid[16]. This group also showed that cyclic adenosine monophosphate (cAMP) increases and reaches a plateau seconds after suspending trout sperm in K+-free medium[97]. Although [K+]o and cAMP were known to influence motility, their relationship is still unknown. Potassium channel blockers like, Tetraethylammonium (TEA+), nonyltriethylammonium1, Ba2+, and Cs+, inhibited sperm motility initiation[98].The above results obtained on induction of sperm motility suggest the hypothesis: ‘‘the inhibition of motility in salmonids is mainly due to K+ ion’’. In other words, membrane hyperpolarization caused directly by transmembrane K+ efflux is the first trigger for initiating sperm motility in salmonid fishes[99].In cyprinids K+ ion also increases sperm velocity and motility and K+ channel inhibitors markedly inhibited the flagellar motion[67, 94, 95]. The potent effects of K+ ion were investigated in demembranated flagella; axonemal motility was found to be directly controlled by the ion concentration[69,70, 71]. It is also clear that the K+ concentrations in diluents used for cryopreservation strongly influence the potential motility of carp sperm[100].To date, the mechanism regulating mobility in sturgeon and paddlefish sperm has not been fully identified, but it presents quite striking similarities with that in salmonid sperm. A recent study by Alavi and his group showed that the potassium concentration in Acipenser persicus seminal plasma was 6.92±0.88 mmol l-1[81]. These findings indicated that seminal plasma K+ is a major inhibitor of sperm motility in A. persicus.
5.3.2. Calcium (Ca2+)
- Sperm motility can be initiated by alteration of the concentration of Ca2+ ions in many species, such as in cyprinids, extracellular Ca2+ ([Ca2+]o) is a prerequisite for the initiation of live sperm motility. Krasznai and his group found that sperm motility is initiated after 30 sec when 10-4 M NaCl was added to the swimming solution[95]. Also, when sperm were demembranated with Triton X-100, they exhibited high motility in 10-6 and 10-5 M Ca2+. Verapamil, a Ca2+ channel blocker inhibited the motility of mature carp sperm prediluted and incubated in physiological solution (140 mM NaCl, 10 mM KCl, 1 mM CaCl2, and 20 mM HEPES, pH 8.5), and completely eliminated the increase in intracellular Ca2+ ([Ca2+]i). They also suggested that influx of [Ca2+]o through specific channels leads to induction of [Ca2+]i release from stores and initiates sperm motility through the calmodulin system. Except verapamil, several specific Ca2+ channel blockers (eg. flunarizine and the conotoxin family) also prevent the increase of [Ca2+]i in the common carp, and the initiation of sperm motility is subsequently suppressed[101]. In tilapia (Oreochromis mossambicus) it was also found that [Ca2+]i is required for activation of sperm motility and can prolong the motility period[102]. The Ca2+-sensitive fluorescent probes have indicated [Ca2+]i increases in single sperm [103] and in sperm populations [90] upon initiation of motility. The contribution of [Ca2+]o and internal Ca2+ stores to the [Ca2+]i increase that occurs when motility is initiated still remains to be established. The river water into which sperm are spawned contains 0.3–0.4 mM Ca2+, enough to contribute to Ca2+ influx through specific sperm plasma membrane Ca2+ channels under physiological conditions.
5.4. Effect of Osmotic Pressure on Sperm Motility
- Exposure to hypo-osmotic or hyperosmotic environment triggers the initiation of fish sperm motility. Sperm of freshwater fish become motile when diluted in a hypotonic solution[16, 67, 88, 104]. Sperm of freshwater Cyprinidae (goldfish, carp, crucian carp and dace) remained immotile when the semen was diluted in solutions of NaCl, KCl, mannitol or glucose iso-osmolar to the seminal plasma (300 mOsm kg-1). The sperm became motile in media containing these solutes if the osmolality was lower than that of the seminal plasma (<200 mOsm kg-1), suggesting that motility is suppressed by the osmolality of the seminal plasma in the sperm duct and initiated by a decrease of osmolality upon spawning into freshwater[67]. Exposure to hyperosmotic seawater also triggers the initiation of sperm motility of marine fish species[16, 105, 106]. Sperm of the marine puffer fish are quiescent in their seminal plasma (around 300 mOsm kg-1) and become motile when there is an increase in the osmolality of the surrounding medium (1200 mOsm kg-1) [105]. This hyperosmotic shock could induce an increase in the [K+]i[106] and in the Ca2+ concentration, and an internal acidification[105]. It has been proposed that these series of variations are the trigger for the activation of motility.For live bearing (internal fertilization) fishes, such as those of the freshwater genus Xiphophorus[107] and the marine ocean pout Macrozoarces americanus[108], sperm motility can be initiated by isotonic osmolalities but not by hypotonic or hypertonic osmolalities. Once initiated, the sperm of these species can remain continuously motile for as long as 1 week[109].Recently, it was found that sperm motility in a euryhaline fish, medaka (Oryzias latipes) was initiated across a broad range of osmolalities varying from deionized water (25 mOsm/kg) and HBSS (Hanks’ balanced salt solution) with hypotonic, isotonic, and hypertonic osmolalities ranging from 92 to 686 mOsm/kg[110]. A euryhaline fish (eg. tilapia), sperm had a similar but attenuated pattern of motility activation. The particular fish studied originated from brackish water and were acclimated to freshwater[102] or seawater[111]. For the freshwater acclimated tilapia, sperm motility could be activated by osmolalities ranging from 0 to 400 mOsm/kg, with or without electrolytes, and Ca2+ was found to be required for motility activation[111]. For the seawater-acclimated tilapia, sperm motility could be activated by osmolalities from 0 to 500 mOsm/kg of NaCl with 10 mM HEPES(N-2-Hydroxyethylpiperazone-n-2-Ethanesulfonic Acid), and the addition of Ca2+ caused increased motility in the presence of high osmolalities (1000 to 1400 mOsm/kg)[111].
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML