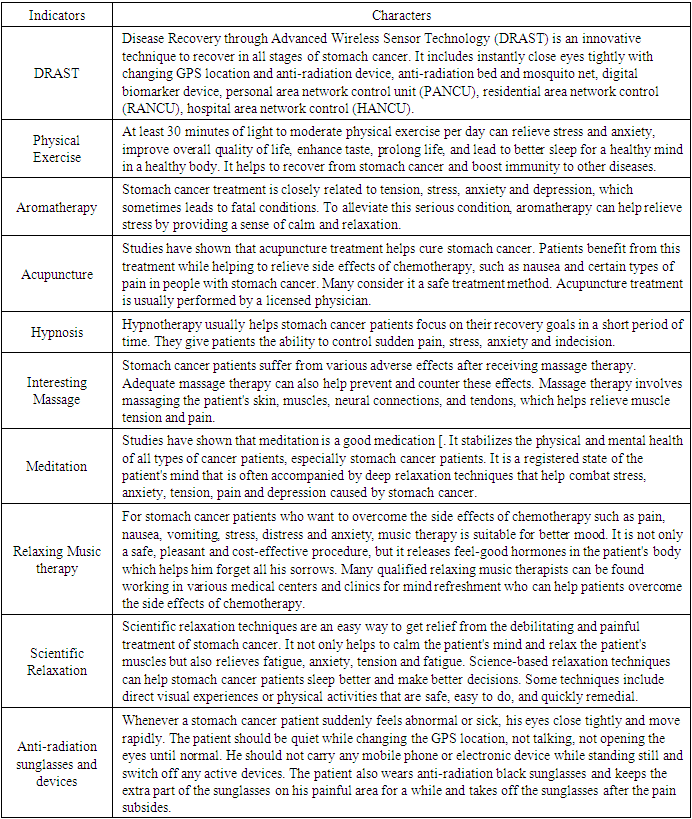
| [1] | Afreen, S., Muthoosamy, K., Manickam, S., & Hashim, U. (2015). Functionalized fullerene (C(6)(0)) as a potential nanomediator in the fabrication of highly sensitive biosensors. Biosensors and Bioelectronics, 63, 354_364. Available from https://doi.org/10.1016/j.bios.2014.07.044. |
| [2] | Ahmad Kiadaliri A, Jarl J, Gavriilidis G, Gerdtham UG. (2013). Alcohol drinking cessation and the risk of laryngeal and pharyngeal cancers: a systematic review and meta-analysis. PLoS One, 8(3): e58158. |
| [3] | Al-Batran SE, Homann N, Pauligk C, Illerhaus G, Martens UM, Stoehlmacher J, et al. (2017). Effect of neoadjuvant chemotherapy followed by surgical resection on survival in patients with limited metastatic gastric or gastroesophageal junction cancer: the AIO-FLOT3 Trial. JAMA Oncol., 3: 1237–44. doi: 10.1001/jamaoncol.2017.0515. |
| [4] | Alwan A, Maclean D, Mandil A. (2001). Assessment of National Capacity for Noncommunicable Disease Prevention and Control; the Report of a Global Survey. Geneva, Switzerland: World Health Organization; 2001. |
| [5] | Arnold M, Pandeya N, Byrnes G, et al. (2015). Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncology, 16(1): 36-46. |
| [6] | Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. (2020). Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int J Mol Sci., 4; 21(11): 4012. doi: 10.3390/ijms21114012. PMID: 32512697; PMCID: PMC7312039. |
| [7] | Piazuelo MB, Epplein M, Correa P. (2010). Gastric cancer: an infectious disease. Infect Dis Clin North Am., 24(4):853-69, vii. doi: 10.1016/j.idc.2010.07.010. PMID: 20937454; PMCID: PMC2954127. |
| [8] | Austoni E, Mirone V, Parazzini F, et al. (2005). Smoking as a risk factor for erectile dysfunction: Data from the Andrology Prevention Weeks 2001–2002. A study of the Italian Society of Andrology (S.I.A.). European Urology, 48(5): 810–818. |
| [9] | Ayob AZ, Ramasamy TS. (2018). Cancer Stem Cells as Key Drivers of Tumour Progression. J BioMed Sci., 25(1): 20. doi: 10.1186/s12929-018-0426-4. |
| [10] | Baetke, S. C., Lammers, T., & Kiessling, F. (2015). Applications of nanoparticles for diagnosis and therapy of cancer. British Journal of Radiology, 88(1054), 20150207. Available from https://doi.org/10.1259/bjr.20150207. |
| [11] | Bagnardi V, Rota M, Botteri E, et al. (2015). Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. British Journal of Cancer, 112(3): 580-593. |
| [12] | Bagnardi V, Rota M, Botteri E, et al. (2013). Light alcohol drinking and cancer: a meta-analysis. Annals of Oncology, 24(2): 301-308. |
| [13] | Bakhshi M, Asadi J, Ebrahimi M, Moradi AV, Hajimoradi M. (2019). Increased Expression of miR-146a, miR-10b, and miR-21 in Cancer Stem-Like Gastro-Spheres. J Cell Biochem., 120(10): 16589–99. doi: 10.1002/jcb.28918. |
| [14] | Bakhtiary, Z., Saei, A. A., Hajipour, M. J., Raoufi, M., Vermesh, O., & Mahmoudi, M. (2016). Targeted superparamagnetic iron oxide nanoparticles for early detection of cancer: Possibilities and challenges. Nanomedicine, 12(2), 287_307. Available from https://doi.org/10.1016/j.nano.2015.10.019. |
| [15] | Bang Y-J, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. (2010). Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet, 376: 687–97. doi: 10.1016/S0140-6736(10)61121-X. |
| [16] | Bao B, Ahmad A, Azmi AS, Ali S, Sarkar FH. (2013). Overview of Cancer Stem Cells (CSCs) and Mechanisms of Their Regulation: Implications for Cancer Therapy. Curr Protoc Pharmacol., Chapter 14: Unit 14 25. doi: 10.1002/0471141755.ph1425s61. |
| [17] | Bao B, Azmi AS, Li Y, Ahmad A, Ali S, Banerjee S, et al. (2014). Targeting CSCs in Tumor Microenvironment: The Potential Role of ROS-Associated miRNAs in Tumor Aggressiveness. Curr Stem Cell Res Ther., 9(1): 22–35. doi: 10.2174/1574888X113089990053. |
| [18] | Barrington WE, Schenk JM, Etzioni R, et al. (2015). Difference in association of obesity with prostate cancer risk between US African American and non-Hispanic white men in the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA Oncology, 1(3): 342-349. |
| [19] | Barton MB, Frommer M, Shafiq J. (2006). Role of radiotherapy in cancer control in low-income and middle-income countries. Lancet Oncology, 7(7): 584–595. [PubMed] |
| [20] | Basati G, Mohammadpour H, Emami Razavi A. (2020). Association of High Expression Levels of SOX2, NANOG, and OCT4 in Gastric Cancer Tumor Tissues with Progression and Poor Prognosis. J Gastrointest Cancer, 51(1): 41–7. doi: 10.1007/s12029-018-00200-x. |
| [21] | Bekaii-Saab T, El-Rayes B. (2017). Identifying and Targeting Cancer Stem Cells in the Treatment of Gastric Cancer. Cancer, 123(8): 1303–12. doi: 10.1002/cncr.30538. |
| [22] | Beom SH, Choi YY, Baek SE, Li SX, Lim JS, Son T, et al. (2018). Multidisciplinary treatment for patients with stage IV gastric cancer: the role of conversion surgery following chemotherapy. BMC Cancer, 18: 1116. doi: 10.1186/s12885-018-4998-x. |
| [23] | Bigarella CL, Liang R, Ghaffari S. (2014). Stem Cells and the Impact of ROS Signaling. Development, 141(22): 4206–18. doi: 10.1242/dev.10708. |
| [24] | Bishayee A. (2014). The role of inflammation and liver cancer. Advances in Experimental Medicine and Biology, 816: 401-435. |
| [25] | Boku N, Yamamoto S, Fukuda H, Shirao K, Doi T, Sawaki A, et al. (2009). Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol., 10:1063–9. doi: 10.1016/S1470-2045(09)70259-1. |
| [26] | Bonequi P, Meneses-Gonzalez F, Correa P, Rabkin CS, Camargo MC. (2013). Risk Factors for Gastric Cancer in Latin America: A Meta-Analysis. Cancer Causes Control., 24(2): 217–31. doi: 10.1007/s10552-012-0110-z. |
| [27] | Bott MJ, Cools-Lartigue J, Tan KS, Dycoco J, Bains MS, Downey RJ, et al. (2018). Safety and feasibility of lung resection after immunotherapy for metastatic or unresectable tumors. Ann Thorac Surg., 106:178– 83. doi: 10.1016/j.athoracsur.2018.02.030. |
| [28] | Brancho D, Tanaka N, Jaeschke A, Ventura JJ, Kelkar N, Tanaka Y, et al. (2003). Mechanism of P38 MAP Kinase Activation In Vivo. Genes Dev., 17(16): 1969–78. doi: 10.1101/gad.1107303. |
| [29] | Bray F, Ferlay J, Soerjomataram I, Siegel R, Torre L, Jemal A. (2018). Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin., 68: 394–24. doi: 10.3322/caac.21492. |
| [30] | Brenner H, Rothenbacher D, Arndt V. (2009). Epidemiology of Stomach Cancer. Methods Mol Biol., 472: 467–77. doi: 10.1007/978-1-60327-492-0_23. |
| [31] | Brinton LA, Cook MB, McCormack V, et al. (2014). Anthropometric and hormonal risk factors for male breast cancer: male breast cancer pooling project results. Journal of the National Cancer Institute, 106(3): djt465. |
| [32] | Bruce N, Rehfuess E, Mehta S, Hutton G, Smith K. (2006). Indoor air pollution. Disease Control Priorities in Developing Countries. 2nd ed. Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, Jha P, Mills A, Musgrove P, editors. New York: Oxford University Press, 793–815. [PubMed] |
| [33] | Brungs D, Aghmesheh M, Vine KL, Becker TM, Carolan MG, Ranson M. (2016). Gastric Cancer Stem Cells: Evidence, Potential Markers, and Clinical Implications. J Gastroenterol., 51(4): 313–26. doi: 10.1007/s00535-015-1125-5. |
| [34] | Burlaka, A. P., Ganusevich, I. I., Gafurov, M. R., Lukin, S. M., & Sidorik, E. P. (2016). Stomach cancer. |
| [35] | Burz, C., Pop, V.-V., Buiga, R., Daniel, S., Samasca, G., Aldea, C., et al. (2018). Circulating tumor cells in clinical research and monitoring patients with colorectal cancer. Oncotarget, 9(36), 24561_24571. Available from https://doi.org/10.18632/oncotarget.25337. |
| [36] | Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. (2003). Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. New England Journal of Medicine, 348(17): 1625-1638. |
| [37] | Campbell PT, Newton CC, Freedman ND, et al. (2016). Body mass index, waist circumference, diabetes, and risk of liver cancer for U.S. adults. Cancer Research, 76(20): 6076-6083. |
| [38] | Cao Y, Willett WC, Rimm EB, Stampfer MJ, Giovannucci EL. (2015). Light to moderate intake of alcohol, drinking patterns, and risk of cancer: results from two prospective US cohort studies. BMJ, 351: h4238. |
| [39] | Chae YC, Angelin A, Lisanti S, Kossenkov AV, Speicher KD, Wang H, et al. (2013). Landscape of the Mitochondrial Hsp90 Metabolome in Tumours. Nat Commun., 4:2139. doi: 10.1038/ncomms3139. |
| [40] | Chan DY, Syn NL, Yap R, Phua JN, Soh TI, Chee CE, et al. (2017). Conversion surgery post-intraperitoneal paclitaxel and systemic chemotherapy for gastric cancer carcinomatosis peritonei. are we ready? J Gastrointest Surg., 21: 425–33. doi: 10.1007/s11605-016-3336-3. |
| [41] | Chao C, Haque R, Caan BJ, et al. (2010). Red wine consumption not associated with reduced risk of colorectal cancer. Nutrition and Cancer, 62(6): 849-855. |
| [42] | Chen CL, Uthaya Kumar DB, Punj V, Xu J, Sher L, Tahara SM, et al. (2016). Nanog Metabolically Reprograms Tumor-Initiating Stem-Like Cells Through Tumorigenic Changes in Oxidative Phosphorylation and Fatty Acid Metabolism. Cell Metab., 23(1): 206–19. doi: 10.1016/j.cmet.2015.12.004. |
| [43] | Chen J, Xie J, Jiang Z, Wang B, Wang Y, Hu X. (2011). Shikonin and its Analogs Inhibit Cancer Cell Glycolysis by Targeting Tumor Pyruvate Kinase-M2. Oncogene, 30(42): 4297–306. doi: 10.1038/onc.2011.137. |
| [44] | Chen WY, Rosner B, Hankinson SE, Colditz GA, Willett WC. (2011). Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA, 306(17): 1884-1890. |
| [45] | Chen Y, Liu L, Wang X, et al. (2013). Body mass index and risk of gastric cancer: a meta-analysis of a population with more than ten million from 24 prospective studies. Cancer Epidemiology, Biomarkers & Prevention, 22(8): 1395-1408. |
| [46] | Chen Y, Wang X, Wang J, Yan Z, Luo J. (2012). Excess body weight and the risk of primary liver cancer: an updated meta-analysis of prospective studies. European Journal of Cancer, 48(14): 2137-2145. |
| [47] | Cheon SH, Rha SY, Jeung HC, Im CK, Kim SH, Kim HR, et al. (2008). Survival benefit of combined curative resection of the stomach (D2 resection) and liver in gastric cancer patients with liver metastases. Ann Oncol., 19: 1146–53. doi: 10.1093/annonc/mdn026. |
| [48] | Chia NY, Tan P. (2016). Molecular Classification of Gastric Cancer. Ann Oncol., 27(5): 763–9. doi: 10.1093/annonc/mdw040. |
| [49] | Chowdhury, S.H., Rashid, M., Miah, M.R., Shahriar, C.S., Tabassum, T., (2021). Effect of Skin Diseases in Modernized Life. American Journal of Dermatology and Venereology, 10 (2), 13-24. doi: 10.5923/j.ajdv.20211002.01.url: http://article.sapub.org/10.5923.j.ajdv.20211002.01.html. |
| [50] | Coalición Multisectorial Peru Contra el Cáncer. (2006). Documento De Consenso. Lima, Peru: Ministerio de Salud; 2006. |
| [51] | Cobb C, Ward KD, Maziak W, Shihadeh AL, Eissenberg T. (2010). Waterpipe tobacco smoking: An emerging health crisis in the United States. American Journal of Health Behavior, 34(3): 275–285. |
| [52] | Collaborative Group on Epidemiological Studies of Ovarian Cancer. (2012). Ovarian cancer and body size: individual participant meta-analysis including 25,157 women with ovarian cancer from 47 epidemiological studies. PLoS Medicine, 9(4): e1001200. |
| [53] | Coller HA. (2019). The Paradox of Metabolism in Quiescent Stem Cells. FEBS Lett., 593(20): 2817–39. doi: 10.1002/1873-3468.13608. |
| [54] | Corey CG, Ambrose BK, Apelberg BJ, King BA. (2015). Flavored tobacco product use among middle and high school students--United States, 2014. MMWR. Morbidity and Mortality Weekly Report, 64(38): 1066-1070. |
| [55] | Cover TL, Peek RMJr. (2013). Diet, Microbial Virulence, and Helicobacter Pylori-Induced Gastric Cancer. Gut Microbes, 4(6): 482–93. doi: 10.4161/gmic.26262. |
| [56] | Darby S, Hill D, Auvinen A, Barros-Dios JM, Baysson H, Bochicchio F, Deo H, Falk R, Forastiere F, Hakama M, Heid I, Kreienbrock L, Kreuzer M, Lagarde F, Makelainen I, Muirhead C, Oberaigner W, Pershagen G, Ruano-Ravina A, Ruosteenoja E, Rosario AS, Tirmarche M, Tomasek L, Whitley E, Wichmann HE, Doll R. (2005). Radon in homes and risk of lung cancer: Collaborative analysis of individual data from 13 European case-control studies. BMJ, 330(7485): 223. [PMC free article] [PubMed] |
| [57] | Darby S, Hill D, Doll R. (2001). Radon: A likely carcinogen at all exposures. Annals of Oncology, 12(10): 1341–1351. |
| [58] | Das B, Pal B, Bhuyan R, Li H, Sarma A, Gayan S, et al. (2019). MYC Regulates the HIF2alpha Stemness Pathway via Nanog and Sox2 to Maintain Self-Renewal in Cancer Stem Cells Versus Non-Stem Cancer Cells. Cancer Res., 79 (16): 4015–25. doi: 10.1158/0008-5472.CAN-18-2847. |
| [59] | Das B, Tsuchida R, Malkin D, Koren G, Baruchel S, Yeger H. (2008). Hypoxia Enhances Tumor Stemness by Increasing the Invasive and Tumorigenic Side Population Fraction. Stem Cells, 26(7): 1818–30. doi: 10.1634/stemcells.2007-0724. |
| [60] | De Francesco EM, Sotgia F, Lisanti MP. (2018). Cancer Stem Cells (CSCs): Metabolic Strategies for Their Identification and Eradication. Biochem J., 475(9): 1611–34. doi: 10.1042/BCJ20170164. |
| [61] | Debas HT, Gosselin R, McCord C, Thind A. (2006). Surgery. Disease Control Priorities in Developing Countries. 2nd ed. Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, Jha P, Mills A, Musgrove P, editors. New York: Oxford University Press, 1245–1259. |
| [62] | DeBerardinis RJ, Chandel NS. (2016). Fundamentals of Cancer Metabolism. Sci Adv., 2(5): e1600200. doi: 10.1126/sciadv.1600200. |
| [63] | Deshmukh A, Deshpande K, Arfuso F, Newsholme P, Dharmarajan A. (2016). Cancer Stem Cell Metabolism: A Potential Target for Cancer Therapy. Mol Cancer, 15(1): 69. doi: 10.1186/s12943-016-0555-x. |
| [64] | Desiderio J, Chao J, Melstrom L, Warner S, Tozzi F, Fong Y, et al. (2017). The 30-year experience-A meta-analysis of randomised and high-quality non-randomised studies of hyperthermic intraperitoneal chemotherapy in the treatment of gastric cancer. Eur J Cancer, 79: 1–14. doi: 0.1016/j.ejca.2017.03.030. |
| [65] | Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, et al. (2009). Association of Reactive Oxygen Species Levels and Radioresistance in Cancer Stem Cells. Nature, 458(7239): 780–3. doi: 10.1038/nature07733. |
| [66] | Ding S, Li C, Cheng N, Cui X, Xu X, Zhou G. (2015). Redox Regulation in Cancer Stem Cells. Oxid Med Cell Longev., 2015:750798. doi: 10.1155/2015/750798. |
| [67] | Dittmar Y, Rauchfuss F, Goetz M, Jandt K, Scheuerlein H, Heise M, et al. (2012). Non-curative gastric resection for patients with stage 4 gastric cancer–a single center experience and current review of literature. Langenbeck’s Arch Surg., 397: 745–53. doi: 10.1007/s00423-012-0902-3. |
| [68] | Dong HM, Wang Q, Wang WL, Wang G, Li XK, Li GD, et al. (2018). A clinical analysis of systemic chemotherapy combined with radiotherapy for advanced gastric cancer. Medicine, 97:e10786. doi: 10.1097/MD.0000000000010786. |
| [69] | Dougan MM, Hankinson SE, Vivo ID, et al. (2015). Prospective study of body size throughout the life-course and the incidence of endometrial cancer among premenopausal and postmenopausal women. International Journal of Cancer, 137(3): 625-37. |
| [70] | Druesne-Pecollo N, Keita Y, Touvier M, et al. (2014). Alcohol drinking and second primary cancer risk in patients with upper aerodigestive tract cancers: a systematic review and meta-analysis of observational studies. Cancer Epidemiology, Biomarkers & Prevention, 23(2): 324-331. |
| [71] | Druesne-Pecollo N, Tehard B, Mallet Y, et al. (2009). Alcohol and genetic polymorphisms: effect on risk of alcohol-related cancer. Lancet Oncology, 10(2): 173-180. |
| [72] | Du R, Hu P, Liu Q, Zhang J. (2019). Conversion surgery for unresectable advanced gastric cancer: a systematic review and meta-analysis. Cancer Investig., 37:16–28. doi: 10.1080/07357907.2018.1551898. |
| [73] | Einama T, Abe H, Shichi S, Matsui H, Kanazawa R, Shibuya K, et al. (2017). Long-term survival and prognosis associated with conversion surgery in patients with metastatic gastric cancer. Mol Clin Oncol., 6: 163–6. doi: 10.3892/mco.2017.1128. |
| [74] | Eisenchlas J. (2006). Cancer Prevention and Management in Latin America. Unpublished paper commissioned by IOM. |
| [75] | El-Sahli S, Xie Y, Wang L, Liu S. (2019). Wnt Signaling in Cancer Metabolism and Immunity. Cancers (Basel), 11(7). doi: 10.3390/cancers11070904. |
| [76] | Epping-Jordan JE, Galea G, Tukuitonga C, Beaglehole R. (2005). Preventing chronic diseases: Taking stepwise action. Lancet, 366(9497):1667–1671. [PubMed] |
| [77] | Falco G, Lee SL, Stanghellini I, Bassey UC, Hamatani T, Ko MS. (2007). Zscan4: A Novel Gene Expressed Exclusively in Late 2-Cell Embryos and Embryonic Stem Cells. Dev Biol., 307(2): 539–50. doi: 10.1016/j.ydbio.2007.05.003. |
| [78] | Fan X, Peters BA, Jacobs EJ, et al. (2018). Drinking alcohol is associated with variation in the human oral microbiome in a large study of American adults. Microbiome, 6(1): 59. |
| [79] | Fedirko V, Tramacere I, Bagnardi V, et al. (2011). Alcohol drinking and colorectal cancer risk: an overall and dose-response meta-analysis of published studies. Annals of Oncology, 22(9): 1958-1972. |
| [80] | Feng HC, Lin JY, Hsu SH, Lan WY, Kuo CS, Tian YF, et al. (2017). Low Folate Metabolic Stress Reprograms DNA Methylation-Activated Sonic Hedgehog Signaling to Mediate Cancer Stem Cell-Like Signatures and Invasive Tumour Stage-Specific Malignancy of Human Colorectal Cancers. Int J Cancer, 141(12): 2537–50. doi: 10.1002/ijc.31008. |
| [81] | Ferlay, J., Shin, H.-R., Bray, F., Forman, D., Mathers, C., Parkin, D.M. (2010). Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer, 127, 2893–2917. doi: 10.1002/ijc.25516. url: https://pubmed.ncbi.nlm.nih.gov/21351269/. |
| [82] | Fitzmaurice C, Akinyemiju TF, Al Lami FH, Alam T, Alizadeh-Navaei R, Allen C, et al. (2018). Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: a systematic analysis for the global burden of disease study. JAMA Oncol., 4: 1553–68. doi: 10.1200/JCO.2018.36.15_suppl.1568. |
| [83] | Flegal KM, Kit BK, Orpana H, Graubard BI. (2013). Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA, 309(1): 71-82. |
| [84] | Fleming ID, Brady LW, Mieszkalski GB, Cooper MR. (1995). Basis for major current therapies for cancer. American Cancer Society. Textbook of Clinical Oncology. 2nd ed. Murphy GP, Lawrence W, Lenhard RE, editors. Atlanta, GA: American Cancer Society, 96. |
| [85] | Forman D, Newell DG, Fullerton F, Yarnell JW, Stacey AR, Wald N, et al. (1991). Association Between Infection With Helicobacter Pylori and Risk of Gastric Cancer: Evidence From a Prospective Investigation. BMJ, 302 (6788):1302–5. doi: 10.1136/bmj.302.6788.1302. |
| [86] | Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, et al. (2014). Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet, 383:31–9. doi: 10.1016/S0140-6736(13)61719-5. |
| [87] | Fujitani K, Yang H-K, Mizusawa J, Kim Y-W, Terashima M, Han S-U, et al. (2016). Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol., 17:309–18. doi: 10.1016/S1470-2045(15)00553-7. |
| [88] | Fukuchi M, Ishiguro T, Ogata K, Suzuki O, Kumagai Y, Ishibashi K, et al. (2015). Prognostic role of conversion surgery for unresectable gastric cancer. Ann Surg Oncol., 22: 3618–24. doi: 10.1245/s10434-015-4422-6. |
| [89] | Fushida S, Fujimura T, Oyama K, Yagi Y, Kinoshita J, Ohta T. (2009). Feasibility and efficacy of preoperative chemotherapy with docetaxel, cisplatin and S-1 in gastric cancer patients with para-aortic lymph node metastases. Anti-cancer Drugs. (2009) 20: 752–6. doi: 10.1097/CAD.0b013e32832ec02b. |
| [90] | Gacci M, Sebastianelli A, Salvi M, et al. (2014). Role of abdominal obesity for functional outcomes and complications in men treated with radical prostatectomy for prostate cancer: results of the Multicenter Italian Report on Radical Prostatectomy (MIRROR) study. Scandinavian Journal of Urology, 48(2): 138-145. |
| [91] | Gallagher EJ, LeRoith D. (2015). Obesity and diabetes: The increased risk of cancer and cancer-related mortality. Physiological Reviews, 95(3): 727-748. |
| [92] | Gao Y, Li J, Xi H, Cui J, Zhang K, Zhang J, et al. (2020). Stearoyl-CoA-desaturase-1 Regulates Gastric Cancer Stem-Like Properties and Promotes Tumour Metastasis Via Hippo/YAP Pathway. Br J Cancer, 122(12): 1837–47. doi: 10.1038/s41416-020-0827-5. |
| [93] | GBD 2016 Alcohol Collaborators. (2018). Alcohol use and burden for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet, doi: 10.1016/S0140-6736(18)31310-2Exit Disclaimer. |
| [94] | Genkinger JM, Spiegelman D, Anderson KE, et al. (2011). A pooled analysis of 14 cohort studies of anthropometric factors and pancreatic cancer risk. International Journal of Cancer, 129(7): 1708-1717. |
| [95] | Glehen O, Gilly FN, Arvieux C, Cotte E, Boutitie F, Mansvelt B, et al. (2010). Peritoneal carcinomatosis from gastric cancer: a multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol., 17: 2370–7. doi: 10.1245/s10434-010-1039-7. |
| [96] | Golestaneh AF, Atashi A, Langroudi L, Shafiee A, Ghaemi N, Soleimani M. (2012). miRNAs Expressed Differently in Cancer Stem Cells and Cancer Cells of Human Gastric Cancer Cell Line MKN-45. Cell Biochem Funct., 30 (5): 411–8. doi: 10.1002/cbf.2815. |
| [97] | Goodwin PJ, Segal RJ, Vallis M, et al. (2014). Randomized trial of a telephone-based weight loss intervention in postmenopausal women with breast cancer receiving letrozole: the LISA trial. Journal of Clinical Oncology, 32(21): 2231-2239. |
| [98] | Goodwin PJ. (2016). Obesity and breast cancer outcomes: How much evidence is needed to change practice? Journal of Clinical Oncology, 34(7): 646-648. Epub 2015 Dec 28. doi: 10.1200/JCO.2015.64.7503Exit Disclaimer. |
| [99] | Gregor MF, Hotamisligil GS. (2011). Inflammatory mechanisms in obesity. Annual Review of Immunology, 29: 415-445. |
| [100] | Grewal P, Viswanathen VA. (2012). Liver cancer and alcohol. Clinics in Liver Disease, 16(4): 839-850. |
| [101] | Guner A, Son T, Cho I, Kwon IG, An JY, Kim H-I, et al. (2016). Liver-directed treatments for liver metastasis from gastric adenocarcinoma: comparison between liver resection and radiofrequency ablation. Gastric Cancer, 19:951–60. doi: 10.1007/s10120-015-0522-z. |
| [102] | Gutierrez-Uzquiza A, Arechederra M, Bragado P, Aguirre-Ghiso JA, Porras A. (2012). p38alpha Mediates Cell Survival in Response to Oxidative Stress via Induction of Antioxidant Genes: Effect on the p70S6K Pathway. J Biol Chem., 287(4): 2632–42. doi: 10.1074/jbc.M111.323709. |
| [103] | Han DS, Suh YS, Kong SH, Lee HJ, Im SA, Bang YJ, et al. (2013). Outcomes of surgery aiming at curative resection in good responder to induction chemotherapy for gastric cancer with distant metastases. J Surg Oncol., 107: 511–6. doi: 10.1002/jso.23284. |
| [104] | Hanai JI, Doro N, Seth P, Sukhatme VP. (2013). ATP Citrate Lyase Knockdown Impacts Cancer Stem Cells In Vitro. Cell Death Dis., 4:e696. doi:10.1038/cddis.2013.215. |
| [105] | Harrigan M, Cartmel B, Loftfield E, et al. (2016). Randomized trial comparing telephone versus in-person weight loss counseling on body composition and circulating biomarkers in women treated for breast cancer: The Lifestyle, Exercise, and Nutrition (LEAN) Study. Journal of Clinical Oncology, 34(7): 669-676. |
| [106] | Hashibe M, Brennan P, Chuang SC, et al. (2009). Interaction between tobacco and alcohol use and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiology, Biomarkers & Prevention, 18(2): 541-550. |
| [107] | Hatsukami DK, Stead LF, Gupta PC. (2008). Tobacco addiction. Lancet, 371(9629):2027–2038. |
| [108] | He W, Liang B, Wang C, Li S, Zhao Y, Huang Q, et al. (2019). MSC-Regulated Lncrna MACC1-AS1 Promotes Stemness and Chemoresistance Through Fatty Acid Oxidation in Gastric Cancer. Oncogene, 38(23): 4637–54. doi: 10.1038/s41388-019-0747-0. |
| [109] | He Z, Li Z, Zhang X, Yin K, Wang W, Xu Z, et al. (2018). MiR-422a Regulates Cellular Metabolism and Malignancy by Targeting Pyruvate Dehydrogenase Kinase 2 in Gastric Cancer. Cell Death Dis., 9(5): 505. doi: 10.1038/s41419-018-0564-3. |
| [110] | Hecht SS. (2003). Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nature Reviews. Cancer, 3(10): 733-744. |
| [111] | Henley SJ, Thun MJ, Chao A, Calle EE. (2004). Association between exclusive pipe smoking and mortality from cancer and other diseases. Journal of the National Cancer Institute, 96(11): 853–861. |
| [112] | Herrera V, Parsonnet J. (2009). Helicobacter Pylori and Gastric Adenocarcinoma. Clin Microbiol Infect., 15(11): 971–6. doi: 10.1111/j.1469-0691.2009.03031.x. |
| [113] | Hossain F, Sorrentino C, Ucar DA, Peng Y, Matossian M, Wyczechowska D, et al. (2018). Notch Signaling Regulates Mitochondrial Metabolism and NF-kappaB Activity in Triple-Negative Breast Cancer Cells via IKKalpha-Dependent Non-Canonical Pathways. Front Oncol., 8:575. doi: 10.3389/fonc.2018.00575. |
| [114] | Hossain, M.S., Miah, M.R., Fardous, M., Ferdous, N.E.J., Mostofa, M.G., Hossain, S.A.M.I., Shahriar, C.S., Talukdar, M.T.H., Ansari, M.A.S. (2021). Histopathological Study of Oral and Oropharyngeal Lesions in a Tertiary Care Hospital. Research In Cancer and Tumor, 9(1), 1-7. doi: 10.5923/j.rct.20210901.01.url: http://article.sapub.org/10.5923.j.rct.20210901.01.html. |
| [115] | Hoyo C, Cook MB, Kamangar F, et al. (2012). Body mass index in relation to oesophageal and oesophagogastric junction adenocarcinomas: a pooled analysis from the International BEACON Consortium. International Journal of Epidemiology, 41(6): 1706-1718. |
| [116] | Hu S-B, Liu C-H, Wang X, Dong Y-W, Zhao L, Liu H-F, et al. (2019)., Pathological evaluation of neoadjuvant chemotherapy in advanced gastric cancer. World JSurg Oncol., 17:3. doi: 10.1186/s12957-018-1534-z. |
| [117] | Hur H, Xuan Y, Kim YB, Lee G, Shim W, Yun J, et al. (2013). Expression of Pyruvate Dehydrogenase Kinase-1 in Gastric Cancer as a Potential Therapeutic Target. Int J Oncol., 42(1): 44–54. doi: 10.3892/ijo.2012.1687. |
| [118] | IARC (International Agency for Research on Cancer). (1997). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Schistosomes, Liver Flukes and Helicobacter pylori. Lyon, France: IARC; 1997. |
| [119] | IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. (2010). Alcohol consumption and ethyl carbamateExit Disclaimer. IARC Monographs on the Evaluation of Carcinogenic Risks in Humans, 96:3-1383. |
| [120] | IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. (2012). Personal habits and indoor combustions. Volume 100 E. A review of human carcinogens. Exit Disclaimer IARC Monographs on the Evaluation of Carcinogenic Risks in Humans, 100(Pt E): 373-472. |
| [121] | IARC. (2004). GLOBOCAN 2002. Lyon, France: IARC; 2004. |
| [122] | Inoue-Choi M, Hartge P, Liao LM, Caporaso N, Freedman ND. (2018). Association between long-term low-intensity cigarette smoking and incidence of smoking-related cancer in the National Institutes of Health-AARP cohort. International Journal of Cancer, 142(2): 271-280. |
| [123] | Inoue-Choi M, Liao LM, Reyes-Guzman C, et al. (2017). Association of long-term, low-intensity smoking with all-cause and cause-specific mortality in the National Institutes of Health-AARP Diet and Health Study. JAMA Internal Medicine, 177(1): 87-95. |
| [124] | International Agency for Research on Cancer. (2012). Tobacco smokingExit Disclaimer, Second-hand tobacco smokeExit Disclaimer, and Smokeless tobaccoExit Disclaimer. In: Personal Habits and Indoor Combustions: A Review of Human Carcinogens. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 100E. Lyon, France: International Agency for Research on Cancer, 43-318. |
| [125] | IOM (Institute of Medicine). (2003). Fulfilling the Potential of Cancer Prevention and Early Detection. Curry SJ, Byers T, Hewitt M, editors. Washington, DC: The National Academies Press; 2003. |
| [126] | IOM. (2004). Meeting Psychosocial Needs of Women with Breast Cancer. Hewitt M, Herdman R, Holland J, editors. Washington, DC: The National Academies Press; 2004. [PubMed] |
| [127] | Ishigami H, Fujiwara Y, Fukushima R, Nashimoto A, Yabusaki H, Imano M, et al. (2018). Phase III Trial Comparing intraperitoneal and intravenous paclitaxel plus S-1 versus cisplatin Plus S-1 in patients with gastric cancer with peritoneal metastasis: PHOENIX-GC Trial. J Clin Oncol. (2018) 36:1922–9. doi: 10.1200/JCO.2018.77.8613. |
| [128] | Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, et al. (2011). CD44 Variant Regulates Redox Status in Cancer Cells by Stabilizing the xCT Subunit of System xc(-) and Thereby Promotes Tumor Growth. Cancer Cell, 19(3): 387–400. doi: 10.1016/j.ccr.2011.01.038. |
| [129] | Ito S, Oki E, Nakashima Y, Ando K, Hiyoshi Y, Ohgaki K, et al. (2015). Clinical significance of adjuvant surgery following chemotherapy for patients with initially unresectable stage IV gastric cancer. Anticancer Res., 35: 401–6. |
| [130] | Ito S, Sano T, Mizusawa J, Takahari D, Katayama H, Katai H, et al. (2017). A phase II study of preoperative chemotherapy with docetaxel, cisplatin, and S-1 followed by gastrectomy with D2 plus para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis: JCOG1002. Gastric Cancer, 20: 322–31. doi: 10.1007/s10120-016-0619-z. |
| [131] | Jagust P, Alcala S, Sainz B, Heeschen C, Sancho P. (2020). Glutathione Metabolism is Essential for Self-Renewal and Chemoresistance of Pancreatic Cancer Stem Cells. World J Stem Cells, 12(11): 1410–28. doi: 10.4252/wjsc.v12.i11.1410. |
| [132] | Jha P, Ramasundarahettige C, Landsman V, et al. (2013). 21st-century hazards of smoking and benefits of cessation in the United States. New England Journal of Medicine, 368(4): 341–350. |
| [133] | Jiang L, Wang H, Li J, Fang X, Pan H, Yuan X, et al. (2014). Up-Regulated FASN Expression Promotes Transcoelomic Metastasis of Ovarian Cancer Cell Through Epithelial-Mesenchymal Transition. Int J Mol Sci., 15 (7): 11539–54. doi: 10.3390/ijms150711539. |
| [134] | Jin L, Alesi GN, Kang S. (2016). Glutaminolysis as a Target for Cancer Therapy. Oncogene, 35(28): 3619–25. doi: 10.1038/onc.2015.447. |
| [135] | Jin L, Li D, Alesi GN, Fan J, Kang HB, Lu Z, et al. (2015). Glutamate Dehydrogenase 1 Signals Through Antioxidant Glutathione Peroxidase 1 to Regulate Redox Homeostasis and Tumor Growth. Cancer Cell, 27(2): 257–70. doi: 10.1016/j.ccell.2014.12.006. |
| [136] | Kanda J, Matsuo K, Suzuki T, et al. (2009). Impact of alcohol consumption with polymorphisms in alcohol-metabolizing enzymes on pancreatic cancer risk in Japanese. Cancer Science, 100(2): 296-302. |
| [137] | Kanda T, Yajima K, Kosugi S, Ishikawa T, Ajioka Y, Hatakeyama K. (2012). Gastrectomy as a secondary surgery for stage IV gastric cancer patients who underwent S-1-based chemotherapy: a multi-institute retrospective study. Gastric Cancer, 15: 235–44. doi: 10.1007/s10120-011-0100-y. |
| [138] | Kennedy NJ, Cellurale C, Davis RJ. (2007). A Radical Role for P38 MAPK in Tumor Initiation. Cancer Cell, 11(2): 101–3. doi: 10.1016/j.ccr.2007.01.009. |
| [139] | Keum N, Greenwood DC, Lee DH, et al. (2015). Adult weight gain and adiposity-related cancers: a dose-response meta-analysis of prospective observational studies. Journal of the National Cancer Institute, 107(2). pii: djv088. |
| [140] | Khan AQ, Ahmed EI, Elareer NR, Junejo K, Steinhoff M, Uddin S. (2019). Role of miRNA-Regulated Cancer Stem Cells in the Pathogenesis of Human Malignancies. Cells, 8(8). doi: 10.3390/cells8080840. |
| [141] | Khazaei S, Rezaeian S, Soheylizad M, Khazaei S, Biderafsh A. (2016). Global Incidence and Mortality Rates of Stomach Cancer and the Human Development Index: An Ecological Study. Asian Pac J Cancer Prev., 17(4): 1701–4. doi: 10.7314/APJCP.2016.17.4.1701. |
| [142] | Kim, HM, Haraguchi N, Ishii H, Ohkuma M, Okano M, Mimori K, et al. (2012). Increased CD13 Expression Reduces Reactive Oxygen Species, Promoting Survival of Liver Cancer Stem Cells via an Epithelial-Mesenchymal Transition-Like Phenomenon. Ann Surg Oncol., 19 Suppl 3:S539–48. doi: 10.1245/s10434-011-2040-5. |
| [143] | Kim SW. (2014). The result of conversion surgery in gastric cancer patients with peritoneal seeding. J Gastr Cancer, 14: 266–70. doi: 10.5230/jgc.2014.14.4.266. |
| [144] | Kinoshita J, Fushida S, Tsukada T, Oyama K, Okamoto K, Makino I, et al. (2015). Efficacy of conversion gastrectomy following docetaxel, cisplatin, and S-1 therapy in potentially resectable stage IV gastric cancer. Eur J Surg Oncol., 41: 1354–60. doi: 10.1016/j.ejso.2015.04.021. |
| [145] | Kitahara CM, Flint AJ, Berrington de Gonzalez A, et al. (2014). Association between class III obesity (BMI of 40-59 kg/m2) and mortality: a pooled analysis of 20 prospective studies. PLoS Medicine, 11(7): e1001673. |
| [146] | Kitahara CM, McCullough ML, Franceschi S, et al. (2016). Anthropometric factors and thyroid cancer risk by histological subtype: Pooled analysis of 22 prospective studies. Thyroid, 26(2): 306-318. |
| [147] | Kitayama J, Ishigami H, Yamaguchi H, Yamashita H, Emoto S, Kaisaki S, et al. (2014). Salvage gastrectomy after intravenous and intraperitoneal paclitaxel (PTX) administration with oral S-1 for peritoneal dissemination of advanced gastric cancer with malignant ascites. Ann Surg Oncol., 21:539–46. doi: 10.1245/s10434-013-3208-y. |
| [148] | Knight JA, Fan J, Malone KE, et al. (2017). Alcohol consumption and cigarette smoking in combination: A predictor of contralateral breast cancer risk in the WECARE study. International Journal of Cancer, 141(5): 916-924. |
| [149] | Knishkowy B, Amitai Y. (2005). Water-pipe (narghile) smoking: An emerging health risk behavior. Pediatrics, 116(1): e113‒119. |
| [150] | Kodera Y, Ito S, Mochizuki Y, Ohashi N, Tanaka C, Kobayashi D, et al. (2012). Long-term follow up of patients who were positive for peritoneal lavage cytology: final report from the CCOG0301 study. Gastric Cancer, 15:335–7. doi: 10.1007/s10120-012-0156-3. |
| [151] | Kodera Y. (2017). Neoadjuvant chemotherapy for gastric adenocarcinoma in Japan. Surg Today, 47: 899–907. doi: 10.1007/s00595-017-1473-2. |
| [152] | Kodera Y. (2018). Surgery with curative intent for stage IV gastric cancer: is it a reality of illusion? Ann Gastroenterol Surg., 2:339–47. doi: 10.1002/ags3.12191. |
| [153] | Koizumi W, Kim YH, Fujii M, Kim HK, Imamura H, Lee KH, et al. (2014). Addition of docetaxel to S-1 without platinum prolongs survival of patients with advanced gastric cancer: a randomized study (START). J Cancer Res Clin Oncol., 140:319–28. doi: 10.1007/s00432-013-1563-5. |
| [154] | Koizumi W, Nakayama N, Tanabe S, Sasaki T, Higuchi K, Nishimura K, et al. (2012). A multicenter phase II study of combined chemotherapy with docetaxel, cisplatin, and S-1 in patients with unresectable or recurrent gastric cancer (KDOG 0601). Cancer Chemotherap Pharmacol., 69: 407–13. doi: 10.1007/s00280-011-1701-1. |
| [155] | Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, et al. (2008). S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol., 9: 215–21. doi: 10.1016/S1470-2045(08)70035-4. |
| [156] | Koukourakis MI, Pitiakoudis M, Giatromanolaki A, Tsarouha A, Polychronidis A, Sivridis E, et al. (2006). Oxygen and Glucose Consumption in Gastrointestinal Adenocarcinomas: Correlation with Markers of Hypoxia, Acidity and Anaerobic Glycolysis. Cancer Sci., 97(10): 1056–60. doi: 10.1111/j.1349-7006.2006.00298.x. |
| [157] | Kwon, OH, Kang TW, Kim JH, Kim M, Noh SM, Song KS, et al. (2012). Pyruvate Kinase M2 Promotes the Growth of Gastric Cancer Cells via Regulation of Bcl-xL Expression at Transcriptional Level. Biochem Biophys Res Commun., 423(1): 38–44. doi: 10.1016/j.bbrc.2012.05.063. |
| [158] | Lamb A, Chen LF. (2013). Role of the Helicobacter Pylori-Induced Inflammatory Response in the Development of Gastric Cancer. J Cell Biochem., 114 (3): 491–7. doi: 10.1002/jcb.24389. |
| [159] | Lamb R, Ozsvari B, Lisanti CL, Tanowitz HB, Howell A, Martinez-Outschoorn UE, et al. (2015). Antibiotics That Target Mitochondria Effectively Eradicate Cancer Stem Cells, Across Multiple Tumor Types: Treating Cancer Like an Infectious Disease. Oncotarget., 6(7): 4569–84. doi: 10.18632/oncotarget.3174. |
| [160] | Laperriere NJ, Bernstein M. (1994). Radiotherapy for brain tumors. CA: A Cancer Journal for Clinicians, 44(2): 96–108. |
| [161] | Lathia JD, Liu H. (2017). Overview of Cancer Stem Cells and Stemness for Community Oncologists. Target Oncol., 12(4): 387–99. doi: 10.1007/s11523-017-0508-3. |
| [162] | Lauby-Secretan B, Scoccianti C, Loomis D, et al. (2016). Body Fatness and Cancer--Viewpoint of the IARC Working Group. New England Journal of Medicine, 375(8): 794-798. doi: 10.1056/NEJMsr1606602Exit Disclaimer. |
| [163] | Lee JE, Hunter DJ, Spiegelman D, et al. (2007). Alcohol intake and renal cell cancer in a pooled analysis of 12 prospective studies. Journal of the National Cancer Institute, 99(10): 801-810. |
| [164] | Lee JH, Paik YH, Lee JS, Song HJ, Ryu KW, Kim CG, et al. (2006). Candidates for curative resection in advanced gastric cancer patients who had equivocal para-aortic lymph node metastasis on computed tomographic scan. Ann Surg Oncol., 13:1163–7. doi: 10.1245/s10434-006-9002-3. |
| [165] | Lee S, Tak E, Lee J, Rashid MA, Murphy MP, Ha J, et al. (2011). Mitochondrial H2O2 Generated From Electron Transport Chain Complex I Stimulates Muscle Differentiation. Cell Res., 21(5): 817–34. doi: 10.1038/cr.2011.55. |
| [166] | Li H, Feng Z, He ML. (2020). Lipid Metabolism Alteration Contributes to and Maintains the Properties of Cancer Stem Cells. Theranostics, 10 (16): 7053–69. doi: 10.7150/thno.41388. |
| [167] | Li L, Gan Y, Li W, Wu C, Lu Z. (2016). Overweight, obesity and the risk of gallbladder and extrahepatic bile duct cancers: A meta-analysis of observational studies. Obesity (Silver Spring), 24(8): 1786-1802. |
| [168] | Li LQ, Yang Y, Chen H, Zhang L, Pan D, Xie WJ. (2016). MicroRNA-181b Inhibits Glycolysis in Gastric Cancer Cells via Targeting Hexokinase 2 Gene. Cancer Biomark, 17(1): 75–81. doi: 10.3233/CBM-160619. |
| [169] | Li W, Jiang H, Yu Y, Wang Y, Wang Z, Cui Y, et al. (2019). Outcomes of gastrectomy following upfront chemotherapy in advanced gastric cancer patients with a single noncurable factor: a cohort study. Cancer Manage Res., 11: 2007–13. doi: 10.2147/CMAR.S192570. |
| [170] | Li X, Wu JB, Li Q, Shigemura K, Chung LW, Huang WC. (2016). SREBP-2 Promotes Stem Cell-Like Properties and Metastasis by Transcriptional Activation of c-Myc in Prostate Cancer. Oncotarget., 7(11): 12869–84. doi: 10.18632/oncotarget.7331. |
| [171] | Lim J, Heo J, Ju H, Shin JW, Kim Y, Lee S, et al. (2020). Glutathione Dynamics Determine the Therapeutic Efficacy of Mesenchymal Stem Cells for Graft- Versus-Host Disease via CREB1-NRF2 Pathway. Sci Adv., 6(16): eaba1334. doi: 10.1126/sciadv.aba1334. |
| [172] | LoConte NK, Brewster AM, Kaur JS, Merrill JK, Alberg AJ. (20118). Alcohol and cancer: A statement of the American Society of Clinical Oncology. Journal of Clinical Oncology, 36(1): 83-93. |
| [173] | Lopez AD, Collishaw NE, Piha T. (1994). A descriptive model of the cigarette epidemic in developed countries. Tobacco Control, 1994(3): 242–247. |
| [174] | Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL. (2006). [eds.]. Global Burden of Disease and Risk Factors. New York: Oxford University Press; 2006. [PubMed] |
| [175] | Lordick F, Siewert JR. (2005). Recent advances in multimodal treatment for gastric cancer: a review. Gastric Cancer, 8:78–85. doi: 10.1007/s10120-005-0321-z. |
| [176] | Ma Y, Yang Y, Wang F, et al. (2013). Obesity and risk of colorectal cancer: a systematic review of prospective studies. PLoS One, 8(1): e53916. |
| [177] | Maeda O, Matsuoka A, Miyahara R, Funasaka K, Hirooka Y, Fukaya M, et al. (2017). Modified docetaxel, cisplatin and capecitabine for stage IV gastric cancer in Japanese patients: a feasibility study. World J Gastroenterol., 23: 1090–7. doi: 10.3748/wjg.v23.i6.1090. |
| [178] | Mahabir S, Leitzmann MF, Virtanen MJ, et al. (2005). Prospective study of alcohol drinking and renal cell cancer risk in a cohort of finnish male smokers. Cancer Epidemiology, Biomarkers & Prevention, 14(1): 170-175. |
| [179] | Mahar AL, Coburn NG, Karanicolas PJ, Viola R, Helyer LK. (2012). Effective palliation and quality of life outcomes in studies of surgery for advanced, non-curative gastric cancer: a systematic review. Gastric Cancer, 15 (Suppl. 1): S138–45. doi: 10.1007/s10120-011-0070-0. |
| [180] | Mari M, Morales A, Colell A, Garcia-Ruiz C, Fernandez-Checa JC. (2009). Mitochondrial Glutathione, a Key Survival Antioxidant. Antioxid Redox Signal, 11(11): 2685–700. doi: 10.1089/ars.2009.2695. |
| [181] | Markar S, Gronnier C, Duhamel A, Pasquer A, Théreaux J, Rieu MCd, et al. (2015). Salvage surgery after chemoradiotherapy in the management of esophageal cancer: is it a viable therapeutic option? J Clin Oncol., 33: 3866–73. doi: 10.1200/JCO.2014.59.9092. |
| [182] | Martin-Romano P, Sola JJ, Diaz-Gonzalez JA, Chopitea A, Iragorri Y, Martinez-Regueira F, et al. (2016). Role of histological regression grade after two neoadjuvant approaches with or without radiotherapy in locally advanced gastric cancer. Br J Cancer, 115: 655–63. doi: 10.1038/bjc.2016.252. |
| [183] | Matsuzaka M, Tanaka R, Sasaki Y. (2016). High Mortality Rate of Stomach Cancer Caused Not by High Incidence But Delays in Diagnosis in Aomori Prefecture, Japan. Asian Pac J Cancer Prev., 17(10): 4723–7. doi: 10.22034/apjcp.2016.17.10.4723. |
| [184] | Mayer MJ, Klotz LH, Venkateswaran V. (2015). Metformin and Prostate Cancer Stem Cells: A Novel Therapeutic Target. Prostate Cancer Prostatic Dis., 18(4): 303–9. doi: 10.1038/pcan.2015.35. |
| [185] | McBride CM, Ostroff JS. (2003). Teachable moments for promoting smoking cessation: The context of cancer care and survivorship. Cancer Control, 10(4): 325–333. |
| [186] | Menendez JA, Lupu R. (2007). Fatty Acid Synthase and the Lipogenic Phenotype in Cancer Pathogenesis. Nat Rev Cancer, 7(10): 763–77. doi: 10.1038/nrc2222. |
| [187] | Meyerhardt JA, Tepper JE, Niedzwiecki D, et al. (2004). Impact of body mass index on outcomes and treatment-related toxicity in patients with stage II and III rectal cancer: findings from Intergroup Trial 0114. Journal of Clinical Oncology, 22(4): 648-657. |
| [188] | Miah, M.R., Hasan, M.M., Hannan, M.A., Parisa, J.T., Uddin, M.J., Uddin, M.B., Rahman, A.A.M.S., Hossain, S.A.M.I., Sharif, M.A., Akhtar, F., Shamsuddin, M.A.S., Alam, M.S.E., Alam, M.S., Abdullah, F., Rahman, M.S., Uddin, M.Be., Shahriar, C.S., Sayok, A.K., Begum, M., Hossain, M.M., Khan, M.S., Ahmed, G., Malik, S.U.F., Samdany, A.A., Ghani, M.A., Hossain, M.S., Nazrin, M.S., Tamim, M.A.K., Selim, M.A., Talukdar, M.T.H., Chowdhury, F.T., Rashid, T.U., Nazim, A.Y.M., Rashid, M., Chowdhury, S.H. (2022a). Global Journal of Health Science, 14(2), 63–112. url: https://ccsenet.org/journal/index.php/gjhs/article/view/0/46717. |
| [189] | Miah, M.R., Hasan, M.M., Parisa, J.T., Alam, M.S.E., Shahriar, C.S., Akhtar, F., Begum, M., Sayok, A.K., Abdullah, F., Shamsuddin, M.A.S. Rahman, A.A.M.S., Alam, M.S., Chowdhury, S.H. et al. (2022). Impact of Oscillated Wireless Sensor Networks to Initiate Cardiac Arrest. International Journal of Internal Medicine, 11(1), 1-17. doi: 10.5923/j.ijim.20221101.01. url: http://article.sapub.org/10.5923.j.ijim.20221101.01.html. |
| [190] | Miah, M.R., Rahman, A.A.M.S., Khan, M.S., Hannan, M.A., Hossain, M.S., Shahriar, C.S., Hossain, S.A.M.I., Talukdar, M.T.H., Samdany, A.A., Alam, M.S., Uddin, M.B., Sayok, A.K., and Chowdhury, S.H. (2021). Effect of Corona Virus Worldwide through Misusing of Wireless Sensor Networks. American Journal of Bioinformatics Research, 11(1), 1-31. url: http://article.sapub.org/10.5923.j.bioinformatics.20211101.01.html. |
| [191] | Mieno H, Yamashita K, Hosoda K, Moriya H, Higuchi K, Azuma M, et al. (2017)., Conversion surgery after combination chemotherapy of docetaxel, cisplatin and S-1 (DCS) for far-advanced gastric cancer. Surgery Today, 47: 1249–58. doi: 10.1007/s00595-017-1512-z. |
| [192] | Miyoshi S, Tsugawa H, Matsuzaki J, Hirata K, Mori H, Saya H, et al. (2018). Inhibiting xCT Improves 5-Fluorouracil Resistance of Gastric Cancer Induced by CD44 Variant 9 Expression. Anticancer Res., 38 (11): 6163–70. doi: 10.21873/anticanres.12969. |
| [193] | Moitra K, Lou H, Dean M. (2011). Multidrug Efflux Pumps and Cancer Stem Cells: Insights Into Multidrug Resistance and Therapeutic Development. Clin Pharmacol Ther., 89(4): 491–502. doi: 10.1038/clpt.2011.14. |
| [194] | Morgagni P, Solaini L, Framarini M, Vittimberga G, Gardini A, Tringali D, et al. (2018). Conversion surgery for gastric cancer: a cohort study from a western center. Int J Surg., 53: 360–5. doi: 10.1016/j.ijsu.2018.04.016. |
| [195] | Mukha A, Dubrovska A. (2020). Metabolic Targeting of Cancer Stem Cells. Front Oncol., 10: 537930. doi: 10.3389/fonc.2020.537930. |
| [196] | Munsell MF, Sprague BL, Berry DA, Chisholm G, Trentham-Dietz A. (2014). Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiologic Reviews, 36: 114-136. |
| [197] | Muto O, Kotanagi H. (2011). Palliative resection for stage IV gastric cancer. J Clin Oncol., 29(4 Suppl): 148. doi: 10.1200/jco.2011.29.4_suppl.148. |
| [198] | Muto O, Kotanagi H. (2012). The efficacy of gastrectomy plus chemotherapy for stage IV gastric cancer. J Clin Oncol., 30(4Suppl):132. doi: 10.1200/jco.2012.30.4_suppl.132. |
| [199] | Nagano O, Okazaki S, Saya H. (2013). Redox Regulation in Stem-Like Cancer Cells by CD44 Variant Isoforms. Oncogene, 32(44):5191–8. doi: 10.1038/onc.2012.638. |
| [200] | Nakai T, Okuno K, Kitaguchi H, Ishikawa H, Yamasaki M. (2013). Unresectable colorectal liver metastases: the safety and efficacy of conversion therapy using hepatic arterial infusion immunochemotherapy with 5-fluorouracil and polyethylene glycol-interferon alpha-2a. World J Surg., 37: 1919–26. doi: 10.1007/s00268-013-2043-4. |
| [201] | Nakajima T, Ota K, Ishihara S, Oyama S, Nishi M, Ohashi Y, et al. (1997). Combined intensive chemotherapy and radical surgery for incurable gastric cancer. Ann Surg Oncol., 4: 203–8. doi: 10.1007/BF02306611. |
| [202] | Nakayama N, Koizumi W, Sasaki T, Higuchi K, Tanabe S, Nishimura K, et al. (2008). A multicenter, phase I dose-escalating study of docetaxel, cisplatin and S-1 for advanced gastric cancer (KDOG0601). Oncology, 75(1–2): 1–7. doi: 10.1159/000151613. |
| [203] | Nash SH, Liao LM, Harris TB, Freedman ND. (2017). Cigarette smoking and mortality in adults aged 70 years and older: Results from the NIH-AARP cohort. American Journal of Preventive Medicine, 52(3): 276-283. |
| [204] | National Cancer Institute. (2004). Staging: Questions and Answers. [accessed October 17, 2006]. Available: http://www.cancer.gov/cancertopics/factsheet/Detection/staging. |
| [205] | National Cancer Institute. (2017). Cancer Trends Progress Report: Secondhand Smoke Exposure. National Institutes of Health, U.S. Department of Health and Human Services, Bethesda, MD, January 2017. |
| [206] | National Center for Health Statistics. (2016). Health, United States, 2015: With Special Feature on Racial Exit Disclaimerand Ethnic Health DisparitiesExit Disclaimer. Hyattsville, MD. |
| [207] | National Toxicology Program. (2016). Tobacco-Related Exposures. In: Report on Carcinogens. Fourteenth Edition. U.S. Department of Health and Human Services, Public Health Service, National Toxicology Program, 2016. |
| [208] | Nelson DE, Jarman DW, Rehm J, et al. (2013). Alcohol-attributable cancer deaths and years of potential life lost in the United States. American Journal of Public Health, 103(4): 641-648. |
| [209] | Neuhouser ML, Aragaki AK, Prentice RL, et al. (2015). Overweight, obesity, and postmenopausal invasive breast cancer risk: A secondary analysis of the Women's Health Initiative randomized clinical trials. JAMA Oncology, 1(5): 611-621. |
| [210] | Niedermaier T, Behrens G, Schmid D, et al. (2015). Body mass index, physical activity, and risk of adult meningioma and glioma: A meta-analysis. Neurology, 85(15): 1342-1350. |
| [211] | Nomura E, Sasako M, Yamamoto S, Sano T, Tsujinaka T, Kinoshita T, et al. (2007). Risk factors for para-aortic lymph node metastasis of gastric cancer from a randomized controlled trial of JCOG9501. Jpn J Clin Oncol., 37: 429–33. doi: 10.1093/jjco/hym067. |
| [212] | O’Connor ML, Xiang D, Shigdar S, Macdonald J, Li Y, Wang T, et al. (2014). Cancer Stem Cells: A Contentious Hypothesis Now Moving Forward. Cancer Lett., 344(2): 180–7. doi: 10.1016/j.canlet.2013.11.012. |
| [213] | Ogden CL, Carroll MD, Kit BK, Flegal KM. (2014). Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA, 311(8):806-814. |
| [214] | Ogden CL, Carroll MD, Lawman HG, et al. (2016). Trends in obesity prevalence among children and adolescents in the United States, 1988-1994 through 2013-2014. JAMA, 315(21): 2292-2299. |
| [215] | Oleastro M, Menard A. (2013). The Role of Helicobacter Pylori Outer Membrane Proteins in Adherence and Pathogenesis. Biol (Basel), 2(3): 1110–34. doi: 10.3390/biology2031110. |
| [216] | Ozsvari B, Sotgia F, Simmons K, Trowbridge R, Foster R, Lisanti MP. (2017). Mitoketoscins: Novel Mitochondrial Inhibitors for Targeting Ketone Metabolism in Cancer Stem Cells (CSCs). Oncotarget., 8(45): 78340–50. doi: 10.18632/oncotarget.21259. |
| [217] | Park IH, Kim SY, Kim YW, Ryu KW, Lee JH, Lee JS, et al. (2011). Clinical characteristics and treatment outcomes of gastric cancer patients with isolated para-aortic lymph node involvement. Cancer Chemotherap Pharmacol., 67: 127–36. doi: 10.1007/s00280-010-1296-y. |
| [218] | Park SM, Li T, Wu S, et al. (2017). Risk of second primary cancer associated with pre-diagnostic smoking, alcohol, and obesity in women with keratinocyte carcinoma. Cancer Epidemiology, 47: 106-113. |
| [219] | Parkin DM. (2005). The global health burden of infection-associated cancers in the year 2002. International Journal of Cancer, 118(12): 3030–3044. [PubMed] |
| [220] | Parsons A, Daley A, Begh R, Aveyard P. (2010). Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: Systematic review of observational studies with meta-analysis. British Medical Journal, 340: b5569. |
| [221] | Paskett ED, Dean JA, Oliveri JM, Harrop JP. (2012). Cancer-related lymphedema risk factors, diagnosis, treatment, and impact: a review. Journal of Clinical Oncology, 30(30): 3726-3733. |
| [222] | Peng F, Wang JH, Fan WJ, Meng YT, Li MM, Li TT, et al. (2018). Glycolysis Gatekeeper PDK1 Reprograms Breast Cancer Stem Cells Under Hypoxia. Oncogene, 37(8): 1062–74. doi: 10.1038/onc.2017.368. |
| [223] | Peng G, Tang Z, Xiang Y, Chen W. (2019). Glutathione Peroxidase 4 Maintains a Stemness Phenotype, Oxidative Homeostasis and Regulates Biological Processes in Panc1 Cancer Stemlike Cells. Oncol Rep., 41(2): 1264–74. doi: 10.3892/or.2018.6905. |
| [224] | Peto R, Darby S, Deo H, et al. (2000). Smoking, smoking cessation, and lung cancer in the U.K. since 1950: Combination of national statistics with two case-control studies. British Medical Journal, 321(7257): 323–329. |
| [225] | Petrick JL, Campbell PT, Koshiol J, et al. (2018). Tobacco, alcohol use and risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: The Liver Cancer Pooling Project. British Journal of Cancer, 118(7): 1005-1012. |
| [226] | Phillips TM, McBride WH, Pajonk F. (2006). The Response of CD24(-/low)/CD44+Breast Cancer-Initiating Cells to Radiation. J Natl Cancer Inst., 98 (24): 1777–85. doi: 10.1093/jnci/djj495. |
| [227] | Piano MR, Benowitz NL, Fitzgerald GA, et al. (2010). Impact of smokeless tobacco products on cardiovascular disease: implications for policy, prevention, and treatment: a policy statement from the American Heart Association. Circulation, 122(15): 1520-1544. doi: 10.1161/CIR.0b013e3181f432c3Exit Disclaimer. |
| [228] | Poli V, Fagnocchi L, Fasciani A, Cherubini A, Mazzoleni S, Ferrillo S, et al. (2018). MYC-Driven Epigenetic Reprogramming Favors the Onset of Tumorigenesis by Inducing a Stem Cell-Like State. Nat Commun., 9 (1): 1024. doi: 10.1038/s41467-018-03264-2. |
| [229] | Portney BA, Arad M, Gupta A, Brown RA, Khatri R, Lin PN, et al. (2020). ZSCAN4 Facilitates Chromatin Remodeling and Promotes the Cancer Stem Cell Phenotype. Oncogene, 39(26): 4970–82. doi: 10.1038/s41388-020-1333-1. |
| [230] | Prignot JJ, Sasco AJ, Poulet E, Gupta PC, Aditama TY. (2008). Alternative forms of tobacco use. International Journal of Tuberculosis and Lung Disease, 12(7): 718–727. |
| [231] | Psaltopoulou T, Sergentanis TN, Ntanasis-Stathopoulos I, et al. (2018). Alcohol consumption and risk of hematological malignancies: A meta-analysis of prospective studies. International Journal of Cancer, 143(3): 486-495. |
| [232] | Radiation Oncology Inquiry. (2002). A Vision for Radiotherapy. Canberra, Australia: Commonwealth of Australia; 2002. |
| [233] | Rai Y, Yadav P, Kumari N, Kalra N, Bhatt AN. (2019). Hexokinase II Inhibition by 3-Bromopyruvate Sensitizes Myeloid Leukemic Cells K-562 to Anti-Leukemic Drug, Daunorubicin. Biosci Rep., 39(9). doi: 10.1042/BSR20190880. |
| [234] | Rajpoot, K., & Jain, S. K. (2021). The role of nanoparticles in the treatment of gastric cancer. Nano Drug Delivery Strategies for the Treatment of Cancers, 165–189. doi:10.1016/b978-0-12-819793-6. |
| [235] | Ramos M, Pereira MA, Charruf AZ, Dias AR, Castria TB, Barchi LC, et al. (2019). Conversion therapy for gastric cancer: expanding the treatment possibilities. Arquivos Brasileiros de Cirurgia Digestiva., 32:e1435. doi: 10.1590/0102-672020190001e1435. |
| [236] | Randi G, Franceschi S, La Vecchia C. (2006). Gallbladder cancer worldwide: geographical distribution and risk factors. International Journal of Cancer, 118(7): 1591-1602. |
| [237] | Rashidkhani B, Akesson A, Lindblad P, Wolk A. (2005). Alcohol consumption and risk of renal cell carcinoma: a prospective study of Swedish women. International Journal of Cancer, 117(5): 848-853. |
| [238] | Raut CP, Posner M, Desai J, Morgan JA, George S, Zahrieh D, et al. (2006). Surgical management of advanced gastrointestinal stromal tumors after treatment with targeted systemic therapy using kinase inhibitors. J Clin Oncol., 24: 2325–31. doi: 10.1200/JCO.2005.05.3439. |
| [239] | Rehm J, Chisholm D, Room R, Lopez AD. (2006). Alcohol. Disease Control Priorities in Developing Countries. 2nd ed. Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, Jha P, Mills A, Musgrove P, editors. New York: Oxford University Press, 887–906. |
| [240] | Rehm J, Patra J, Popova S. (2007). Alcohol drinking cessation and its effect on esophageal and head and neck cancers: a pooled analysis. International Journal of Cancer, 121(5): 1132-1137. |
| [241] | Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. (2008). Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet, 371(9612): 569-578. |
| [242] | Roberts DL, Dive C, Renehan AG. (2010). Biological mechanisms linking obesity and cancer risk: new perspectives. Annual Review of Medicine, 61: 301–316. |
| [243] | Robles-Flores M, Moreno-Londono AP, Castaneda-Patlan MC. (2021). Signaling Pathways Involved in Nutrient Sensing Control in Cancer Stem Cells: An Overview. Front Endocrinol (Lausanne), 12:627745. doi: 10.3389/fendo.2021.627745. |
| [244] | Roos DE, Turner SL, O’Brien PC, Smith JG, Spry NA, Burmeister BH, Hoskin PJ, Ball DL. (2005). Trans-Tasman Radiation Oncology Group. Randomized trial of 8 Gy in 1 versus 20 Gy in 5 fractions of radiotherapy for neuropathic pain due to bone metastases. Radiotherapy & Oncology, 75(1): 54–63. [PubMed] |
| [245] | Rosenstock L, Cullen M, Fingerhut M. (2006). Occupational health. Disease Control Priorities in Developing Countries. 2nd ed. Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, Jha P, Mills A, Musgrove P, editors. New York: Oxford University Press, 127–1145. [PubMed] |
| [246] | Roviello F, Pedrazzani C, Marrelli D, Di Leo A, Caruso S, Giacopuzzi S, et al. (2010). Super-extended (D3) lymphadenectomy in advanced gastric cancer. Eur J Surg Oncol., 36:439–46. doi: 10.1016/j.ejso.2010.03.008. |
| [247] | Ruggieri V, Russi S, Zoppoli P, La Rocca F, Angrisano T, Falco G, et al. (2019). The Role of MicroRNAs in the Regulation of Gastric Cancer Stem Cells: A Meta-Analysis of the Current Status. J Clin Med., 8(5). doi: 10.3390/jcm8050639. |
| [248] | Russi S, Verma HK, Laurino S, Mazzone P, Storto G, Nardelli A, et al. (2019). Adapting and Surviving: Intra and Extra-Cellular Remodeling in Drug-Resistant Gastric Cancer Cells. Int J Mol Sci., 20(15). doi: 10.3390/ijms20153736. |
| [249] | Saito M, Kiyozaki H, Takata O, Suzuki K, Rikiyama T. (2014). Treatment of stage IV gastric cancer with induction chemotherapy using S-1 and cisplatin followed by curative resection in selected patients. World J Surg Oncol., 12:406. doi: 10.1186/1477-7819-12-406. |
| [250] | Sakamoto Y, Sano T, Shimada K, Esaki M, Saka M, Fukagawa T, et al. (2007). Favorable indications for hepatectomy in patients with liver metastasis from gastric cancer. J Surg Oncol., 95: 534–9. doi: 10.1002/jso.20739. |
| [251] | Sancho P, Barneda D, Heeschen C. (2016). Hallmarks of Cancer Stem Cell Metabolism. Br J Cancer, 114(12): 1305–12. doi: 10.1038/bjc.2016.152. |
| [252] | Sanfilippo KM, McTigue KM, Fidler CJ, et al. (2014). Hypertension and obesity and the risk of kidney cancer in 2 large cohorts of US men and women. Hypertension, 63(5): 934-41. |
| [253] | Sano T, Aiko T. (2011). New Japanese classifications and treatment guidelines for gastric cancer: revision concepts and major revised points. Gastric Cancer, 14: 97–100. doi: 10.1007/s10120-011-0040-6. |
| [254] | Santos CR, Schulze A. (2012). Lipid Metabolism in Cancer. FEBS J., 279 (15): 2610–23. doi: 10.1111/j.1742-4658.2012.08644.x. |
| [255] | Sato Y, Ohnuma H, Nobuoka T, Hirakawa M, Sagawa T, Fujikawa K, et al. (2017). Conversion therapy for inoperable advanced gastric cancer patients by docetaxel, cisplatin, and S-1 (DCS) chemotherapy: a multi-institutional retrospective study. Gastric Cancer, 20: 517–26. doi: 10.1007/s10120-016-0633-1. |
| [256] | Sato Y, Takayama T, Sagawa T, Takahashi Y, Ohnuma H, Okubo S, et al. (2010). Phase II study of S-1, docetaxel and cisplatin combination chemotherapy in patients with unresectable metastatic gastric cancer. Cancer Chemotherap Pharmacol., 66:721–8. doi: 10.1007/s00280-009-1215-2. |
| [257] | Satoh S, Okabe H, Teramukai S, Hasegawa S, Ozaki N, Ueda S, et al. (2012). Phase II trial of combined treatment consisting of preoperative S-1 plus cisplatin followed by gastrectomy and postoperative S-1 for stage IV gastric cancer. Gastric Cancer. (2012) 15: 61–9. doi: 10.1007/s10120-011-0066-9. |
| [258] | Sautner T, Hofbauer F, Depisch D, Schiessel R, Jakesz R.(1994). Adjuvant intraperitoneal cisplatin chemotherapy does not improve long-term survival after surgery for advanced gastric cancer. J Clin Oncol., 12: 970–4. doi: 10.1200/JCO.1994.12.5.970. |
| [259] | Schieber M, Chandel NS. (2014). ROS Function in Redox Signaling and Oxidative Stress. Curr Biol., 24(10): R453–62. doi: 10.1016/j.cub.2014.03.034. |
| [260] | Schmitz KH, Neuhouser ML, Agurs-Collins T, et al. (2013). Impact of obesity on cancer survivorship and the potential relevance of race and ethnicity. Journal of the National Cancer Institute, 105(18): 1344-1354. |
| [261] | Seo BR, Bhardwaj P, Choi S. (2015). Obesity-dependent changes in interstitial ECM mechanics promote breast tumorigenesis. Science Translational Medicine, 7(301): 301ra130. |
| [262] | Setiawan VW, Yang HP, Pike MC, et al. (2013). Type I and II endometrial cancers: have they different risk factors? Journal of Clinical Oncology, 31(20): 2607-2618. |
| [263] | Shaban S, El-Husseny MWA, Abushouk AI, Salem AMA, Mamdouh M, Abdel-Daim MM. (2017). Effects of Antioxidant Supplements on the Survival and Differentiation of Stem Cells. Oxid Med Cell Longev, 2017: 5032102. doi: 10.1155/2017/5032102. |
| [264] | Shao M, Zhang J, Zhang J, Shi H, Zhang Y, Ji R, et al. (2020). SALL4 Promotes Gastric Cancer Progression via Hexokinase II Mediated Glycolysis. Cancer Cell Int., 20: 188. doi: 10.1186/s12935-020-01275-y. |
| [265] | Sheflin AM, Whitney AK, Weir TL. (2014). Cancer-promoting effects of microbial dysbiosis. Current Oncology Reports, 16(10): 406. |
| [266] | Shen L, Shan YS, Hu HM, Price TJ, Sirohi B, Yeh KH, et al. (2013). Management of gastric cancer in Asia: resource-stratified guidelines. Lancet Oncol., 14: e535–47. doi: 10.1016/S1470-2045(13)70436-4. |
| [267] | Shibuya K, Okada M, Suzuki S, Seino M, Seino S, Takeda H, et al. Targeting the Facilitative Glucose Transporter GLUT1 Inhibits the Self-Renewal and Tumor-Initiating Capacity of Cancer Stem Cells. Oncotarget (2015) 6 (2): 651–61. doi: 10.18632/oncotarget.2892. |
| [268] | Shin SJ, Chun SH, Kim KO, Kim MK, Lee KH, Hyun MS, et al. (2005). The efficacy of paclitaxel and cisplatin combination chemotherapy for the treatment of metastatic or recurrent gastric cancer: a multicenter phase II study. Korean J Int Med., 20: 135–40. doi: 10.3904/kjim.2005.20.2.135. |
| [269] | Shiroki T, Yokoyama M, Tanuma N, Maejima R, Tamai K, Yamaguchi K, et al. (2017). Enhanced Expression of the M2 Isoform of Pyruvate Kinase Is Involved in Gastric Cancer Development by Regulating Cancer-Specific Metabolism. Cancer Sci., 108(5): 931–40. doi: 10.1111/cas.13211. |
| [270] | Shitara K, Ohtsu A. (2016). Advances in systemic therapy for metastatic or advanced gastric cancer. J Natl Comp Cancer Netw., 14:1313–20. doi: 10.6004/jnccn.2016.0138. |
| [271] | Shitara K. (2017). Chemotherapy for advanced gastric cancer: future perspective in Japan. Gastric Cancer, 20 (Suppl 1): 102–10. doi: 10.1007/s10120-016-0648-7. |
| [272] | Simapivapan P, Boltong A, Hodge A. (2016). To what extent is alcohol consumption associated with breast cancer recurrence and second primary breast cancer? A systematic review. Cancer Treatment Reviews, 50: 155-167. |
| [273] | Sitarz R, Skierucha M, Mielko J, Offerhaus GJA, Maciejewski R, Polkowski WP. (2018). Gastric Cancer: Epidemiology, Prevention, Classification, and Treatment. Cancer Manag Res., 10:239–48. doi: 10.2147/CMAR.S149619 7. |
| [274] | Sivan A, Corrales L, Hubert N, et al. (2015). Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science, 350(6264): 1084-1089. |
| [275] | Smith-Simone S, Maziak W, Ward KD, Eissenberg T. (2008). Waterpipe tobacco smoking: Knowledge, attitudes, beliefs, and behavior in two U.S. samples. Nicotine Tobacco Research, 10(2): 393–398. |
| [276] | Snyder V, Reed-Newman TC, Arnold L, Thomas SM, Anant S. (2018). Cancer Stem Cell Metabolism and Potential Therapeutic Targets. Front Oncol., 8:203. doi: 10.3389/fonc.2018.00203. |
| [277] | Solaini L, Ministrini S, Bencivenga M, D’Ignazio A, Marino E, Cipollari C, et al. (2019). Conversion gastrectomy for stage IV unresectable gastric cancer: a GIRCG retrospective cohort study. Gastric Cancer, 22: 1285–93. doi: 10.1007/s10120-019-00968-2. |
| [278] | Song M, Lee H, Nam MH, Jeong E, Kim S, Hong Y, et al. (2017). Loss-of-Function Screens of Druggable Targetome Against Cancer Stem-Like Cells. FASEB J., 31(2): 625–35. doi: 10.1096/fj.201600953. |
| [279] | Stewart BW, Kleihues P. (2003). World Cancer Report. Lyon, France: IARC Press; 2003. |
| [280] | Stornetta A, Guidolin V, Balbo S. (2018). Alcohol-derived acetaldehyde exposure in the oral cavity. Cancers, 10(1). pii: E20. |
| [281] | Sugarbaker PH. (2016). Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of gastrointestinal cancers with peritoneal metastases: progress toward a new standard of care. Cancer Treat Rev., 48:42–9. doi: 10.1016/j.ctrv.2016.06.007. |
| [282] | Sun J, Song Y, Wang Z, Chen X, Gao P, Xu Y, et al. (2013). Clinical significance of palliative gastrectomy on the survival of patients with incurable advanced gastric cancer: a systematic review and meta-analysis. BMC Cancer, 13: 577. doi: 10.1186/1471-2407-13-577. |
| [283] | Sun X, Jiao X, Pestell TG, Fan C, Qin S, Mirabelli E, et al. (2014). MicroRNAs and Cancer Stem Cells: The Sword and the Shield. Oncogene, 33(42): 4967–77. doi: 10.1038/onc.2013.492. |
| [284] | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. (2021). Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin., 3(71): 209–49. doi: 10.3322/caac.21660. |
| [285] | Suzuki T, Tanabe K, Taomoto J, Yamamoto H, Tokumoto N, Yoshida K, et al. (2010). Preliminary trial of adjuvant surgery for advanced gastric cancer. Oncol Lett., 1:743–7. doi: 10.3892/ol_00000130. |
| [286] | Tagliaferri D, Mazzone P, Noviello TMR, Addeo M, Angrisano T, Del Vecchio L, et al. (2019). Retinoic Acid Induces Embryonic Stem Cells (ESCs) Transition to 2 Cell-Like State Through a Coordinated Expression of Dux and Duxbl1. Front Cell Dev Biol., 7: 385. doi: 10.3389/fcell.2019.00385. |
| [287] | Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. (2007). Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell, 131(5): 861–72. doi: 10.1016/j.cell.2007.11.019 26. |
| [288] | Takahashi RU, Miyazaki H, Ochiya T. (2014). The Role of microRNAs in the Regulation of Cancer Stem Cells. Front Genet., 4: 295. doi: 10.3389/fgene.2013.00295. |
| [289] | Takaishi S, Okumura T, Tu S, Wang SS, Shibata W, Vigneshwaran R, et al. (2009). Identification of Gastric Cancer Stem Cells Using the Cell Surface Marker CD44. Stem Cells, 27(5):1006–20. doi: 10.1002/stem.30. |
| [290] | Tan P, Yeoh KG. (2015). Genetics and Molecular Pathogenesis of Gastric Adenocarcinoma. Gastroenterology, 149(5): 1153–62.e3. doi: 10.1053/j.gastro.2015.05.059. |
| [291] | Tatsuzaki H, Levin CV. (2001). Quantitative status of resources for radiation therapy in Asia and Pacific region. Radiotherapy & Oncology, 60(1): 81–89. [PubMed] |
| [292] | Tee MC, Cao Y, Warnock GL, Hu FB, Chavarro JE. (2013). Effect of bariatric surgery on oncologic outcomes: a systematic review and meta-analysis. Surgical Endoscopy, 27(12): 4449-4456. |
| [293] | Teras LR, Kitahara CM, Birmann BM, et al. (2014). Body size and multiple myeloma mortality: a pooled analysis of 20 prospective studies. British Journal of Haematology, 166(5): 667-676. |
| [294] | Terashima M. (2016). Conversion therapy for gastric cancer: who can make conversion as successful as Goromaru? Gastric Cancer, 19:685–6. doi: 10.1007/s10120-016-0609-1. |
| [295] | Thomassen I, van Gestel YR, van Ramshorst B, Luyer MD, Bosscha K, Nienhuijs SW, et al. (2014). Peritoneal carcinomatosis of gastric origin: a population-based study on incidence, survival and risk factors. Int J Cancer, 134:622–8. doi: 10.1002/ijc.28373. |
| [296] | Thorens B, Mueckler M. (2010). Glucose Transporters in the 21st Century. Am J Physiol Endocrinol Metab., 298(2): E141–5. doi: 10.1152/ajpendo.00712.2009. |
| [297] | Tormos KV, Anso E, Hamanaka RB, Eisenbart J, Joseph J, Kalyanaraman B, et al. (2011). Mitochondrial Complex III ROS Regulate Adipocyte Differentiation. Cell Metab., 14(4): 537–44. doi: 10.1016/j.cmet.2011.08.007. |
| [298] | Tramacere I, Pelucchi C, Bonifazi M, et al. (2012). Alcohol drinking and non-Hodgkin lymphoma risk: a systematic review and a meta-analysis. Annals of Oncology, 23(11): 2791-2798. |
| [299] | Travis LB, Rabkin CS, Brown LM, et al. (2006). Cancer survivorship―genetic susceptibility and second primary cancers: Research strategies and recommendations. Journal of the National Cancer Institute, 98(1):15–25. |
| [300] | Troiano A, Pacelli C, Ruggieri V, Scrima R, Addeo M, Agriesti F, et al. (2020). ZSCAN4(+) Mouse Embryonic Stem Cells Have an Oxidative and Flexible Metabolic Profile. EMBO Rep., 21(6): e48942. doi: 10.15252/embr.201948942. |
| [301] | Tsuburaya A, Mizusawa J, Tanaka Y, Fukushima N, Nashimoto A, Sasako M. (2014). Neoadjuvant chemotherapy with S-1 and cisplatin followed by D2 gastrectomy with para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis. Br J Surg., 101:653–60. doi: 10.1002/bjs.9484. |
| [302] | Tsuchida R, Das B, Yeger H, Koren G, Shibuya M, Thorner PS, et al. (2008). Cisplatin Treatment Increases Survival and Expansion of a Highly Tumorigenic Side Population Fraction by Upregulating VEGF/Flt1 Autocrine Signaling. Oncogene, 27(28): 3923–34. doi: 10.1038/onc.2008.38. |
| [303] | Turati F, Garavello W, Tramacere I, et al. (2013). A meta-analysis of alcohol drinking and oral and pharyngeal cancers: results from subgroup analyses. Alcohol and Alcoholism, 48(1): 107-118. |
| [304] | U.S. Department of Health and Human Services. (1990). The Health Benefits of Smoking Cessation: A Report of the Surgeon GeneralExit Disclaimer. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 1990. |
| [305] | U.S. Department of Health and Human Services. (2004). The Health Consequences of Smoking: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2004. |
| [306] | U.S. Department of Health and Human Services. (2006). The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Rockville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2006. |
| [307] | U.S. Department of Health and Human Services. (2010). How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2010. |
| [308] | U.S. Department of Health and Human Services. (2014). The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General, 2014. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014. |
| [309] | U.S. Environmental Protection Agency. (1992). Respiratory Health Effects of Passive Smoking: Lung Cancer and Other Disorders. Washington, DC: U.S. Environmental Protection Agency, Office of Health and Environmental Assessment, Office of Research and Development; 1992. |
| [310] | Uddin, M.B., Hoque, M., Ali, M.M., Islam, A., Miah, M.R. (2021). Abandonment and Outcome of Induction Chemotherapy in Childhood Acute Lymphoblastic Leukemia, Research In Cancer and Tumor, 9(1), 8-14. doi: 10.5923/j.rct.20210901.02. url: http://article.sapub.org/10.5923.j.rct.20210901.02.html. |
| [311] | Uemura N, Kikuchi S, Sato Y, Ohnuma H, Okamoto K, Miyamoto H, et al. (2017). A phase II study of modified docetaxel, cisplatin, and S-1 (mDCS) chemotherapy for unresectable advanced gastric cancer. Cancer Chemotherap Pharmacol., 80:707–13. doi: 10.1007/s00280-017-3404-8. |
| [312] | Ustaalioglu BBO, Bilici A, Tilki M, Surmelioglu A, Erkol B, Figen M, et al. (2018). Capecitabine-cisplatin versus 5-fluorouracil/leucovorin in combination with radiotherapy for adjuvant therapy of lymph node positive locally advanced gastric cancer. J Cancer Res Therap., 14 (Supplement): S736–41. doi: 10.4103/0973-1482.183548. |
| [313] | Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. (2006). Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol., 24:4991–7. doi: 10.1200/JCO.2006.06.8429. |
| [314] | Van Der Giessen PH, Alert J, Badri C, Bistrovic M, Deshpande D, Kardamakis D, Van Der Merwe D, Da Motta N, Pinillos L, Sajjad R, Tian Y, Levin V. (2004). Multinational assessment of some operational costs of teletherapy. Radiotherapy & Oncology, 71(3): 347–355. [PubMed] |
| [315] | Vartolomei MD, Kimura S, Ferro M, et al. (2018). The impact of moderate wine consumption on the risk of developing prostate cancer. Clinical Epidemiology, 10: 431-444. |
| [316] | Verma HK, Ratre YK, Mazzone P, Laurino S, Bhaskar LVKS. (2020). Micro-RNA Facilitated Chemoresistance in Gastric Cancer: A Novel Biomarkers and Potential Therapeutics. Alexandria J Med., 56(1): 81–92. doi: 10.1080/20905068.2020.1779992. |
| [317] | Vétizou M, Pitt JM, Daillère R, et al. (2015). Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science, 350(6264): 1079-1084. |
| [318] | Villanti AC, Richardson A, Vallone DM, Rath JM. (2013). Flavored tobacco product use among U.S. young adults. American Journal of Preventive Medicine, 44(4): 388-391. |
| [319] | Visweswaran M, Arfuso F, Warrier S, Dharmarajan A. (2020). Aberrant Lipid Metabolism as an Emerging Therapeutic Strategy to Target Cancer Stem Cells. Stem Cells, 38(1):6–14. doi: 10.1002/stem.3101. |
| [320] | Wallin A, Larsson SC. (2011). Body mass index and risk of multiple myeloma: a meta-analysis of prospective studies. European Journal of Cancer, 47(11):1606-1615. |
| [321] | Walter K, Hong SM, Nyhan S, Canto M, Fedarko N, Klein A, et al. (2009). Serum Fatty Acid Synthase as a Marker of Pancreatic Neoplasia. Cancer Epidemiol Biomarkers Prev (2009) 18(9): 2380–5. doi: 10.1158/1055-9965.EPI-09-0144. |
| [322] | Walter V, Jansen L, Hoffmeister M, Brenner H. (2014). Smoking and survival of colorectal cancer patients: systematic review and meta-analysis. Annals of Oncology, 25(8): 1517–1525. |
| [323] | Wang F, Xu Y. (2014). Body mass index and risk of renal cell cancer: a dose-response meta-analysis of published cohort studies. International Journal of Cancer, 135(7): 1673-86. |
| [324] | Wang KC, Chang HY. (2011). Molecular Mechanisms of Long Noncoding RNAs. Mol Cell, 43(6): 904–14. doi: 10.1016/j.molcel.2011.08.018. |
| [325] | Wang L, Zhang T, Wang L, Cai Y, Zhong X, He X, et al. (2017). Fatty Acid Synthesis is Critical for Stem Cell Pluripotency via Promoting Mitochondrial Fission. EMBO J., 36(10): 1330–47. doi: 10.15252/embj.201695417. |
| [326] | Warren GW, Kasza KA, Reid ME, Cummings KM, Marshall JR. (2013). Smoking at diagnosis and survival in cancer patients. International Journal of Cancer, 132(2): 401–410. |
| [327] | Watanabe Y, Suefuji H, Hirose Y, Kaida H, Suzuki G, Uozumi J, et al. (2013). 18F-FDG Uptake in Primary Gastric Malignant Lymphoma Correlates with Glucose Transporter 1 Expression and Histologic Malignant Potential. Int J Hematol., 97(1): 43–9. doi: 10.1007/s12185-012-1225-4. |
| [328] | Wei MF, Chen MW, Chen KC, Lou PJ, Lin SY, Hung SC, et al. (2014). Autophagy Promotes Resistance to Photodynamic Therapy-Induced Apoptosis Selectively in Colorectal Cancer Stem-Like Cells. Autophagy, 10 (7):1179–92. doi: 10.4161/auto.28679. |
| [329] | White AJ, DeRoo LA, Weinberg CR, Sandler DP. (2017). Lifetime alcohol intake, binge drinking behaviors, and breast cancer risk. American Journal of Epidemiology, 186(5): 541-549. |
| [330] | Whiteman DC, Wilson LF. (2016). The fractions of cancer attributable to modifiable factors: A global review. Cancer Epidemiology, 44:203-221. |
| [331] | WHO (World Health Organization). (1996). Cancer Pain Relief. 2nd edition. Geneva, Switzerland: WHO; 1996. |
| [332] | WHO. (2002). National Cancer Control Programmes: Policies and Managerial Guidelines. 2nd edition. Geneva, Switzerland: WHO; 2002. |
| [333] | WHO. (2003). INTERSUN: The Global UV Project, A Guide and Compendium. Geneva, Switzerland: WHO; 2003. |
| [334] | WHO. (2004). Global Strategy on Diet, Physical Activity and Health. [accessed October 17, 2006]. Available: http://www.who.int/dietphysicalactivity/goals/en/. |
| [335] | WHO. (2005). Preventing Chronic Diseases: A Vital Investment. Geneva, Switzerland: WHO; 2005. |
| [336] | WHO. (2005a). Essential Medicines: WHO Model List, 14th edition. [Online]. 2005. [accessed January 28, 2006]. Available: http://whqlibdoc.who.int/hq/2005/a87017_eng.pdf. |
| [337] | WHO. (2006) Essential Medicines. [accessed July 5, 2006]. Available: http://www.who.int/medicines/services/essmedicines_def/en/index.html. |
| [338] | WHO. (2006a). Ultraviolet Radiation and the INTERSUN Programme. [Online]. [accessed October 16, 2006]. Available: http://www.who.int/uv/intersunprogramme/en/. |
| [339] | WHO. (2015). World Health Organization (Tobacco Free Initiative): Advisory Note. Waterpipe Tobacco Smoking: Health Effects, Research Needs and Recommended Actions for Regulators, 2nd editionExit Disclaimer. 2015. (retrieved December 12, 2017, from http://www.who.int/tobacco/publications/prod_regulation/waterpipesecondedition/en/Exit Disclaimer). |
| [340] | WHO. Cancer Control: Knowledge Into Action. WHO Guide for Effective Programmes (Six Volumes). Geneva, Switzerland: World Health Organization; Forthcoming. |
| [341] | Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, et al. (2014). Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol., 15:1224–35. doi: 10.1016/S1470-2045(14) 70420-6. |
| [342] | Wise DR, Thompson CB. (2010). Glutamine Addiction: A New Therapeutic Target in Cancer. Trends Biochem Sci., 35(8): 427–33. doi: 10.1016/j.tibs. 2010.05.003. |
| [343] | World Cancer Research Fund International/American Institute for Cancer Research. (2015). Continuous Update Project Report: Diet, Nutrition, Physical Activity and Gallbladder Cancer. Available at http://www.wcrf.org/sites/default/files/Gallbladder-Cancer-2015-Report.pdfExit Disclaimer. |
| [344] | World Cancer Research Fund. (1997). Food, Nutrition, and the Prevention of Cancer: A Global Perspective. Washington, DC: American Institute for Cancer Research. |
| [345] | World Health Assembly. (2005). Cancer prevention and control. WHA Resolution 58.22. Geneva, Switzerland: WHO; 2005. |
| [346] | Wu, C., Wang, Z., Song, X., et al. (2014). Joint analysis of three genome-wide association studies of esophageal squamous cell carcinoma in Chinese populations. Nature Genetics, 46(9): 1001-1006. |
| [347] | Wu, J., Hu L, Wu F, Zou L, He T. (2017). Poor Prognosis of Hexokinase 2 Overexpression in Solid Tumors of Digestive System: A Meta-Analysis. Oncotarget., 8(19): 32332–44. doi: 10.18632/oncotarget.15974. |
| [348] | Wyss A, Hashibe M, Chuang SC, et al. (2013). Cigarette, cigar, and pipe smoking and the risk of head and neck cancers: Pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. American Journal of Epidemiology, 178(5): 679-690. |
| [349] | Xiang Y, Stine ZE, Xia J, Lu Y, O’Connor RS, Altman BJ, et al. (2015). Targeted Inhibition of Tumor-Specific Glutaminase Diminishes Cell-Autonomous Tumorigenesis. J Clin Invest., 125(6): 2293–306. doi: 10.1172/JCI75836. |
| [350] | Yadav UP, Singh T, Kumar P, Sharma P, Kaur H, Sharma S, et al. (2020). Metabolic Adaptations in Cancer Stem Cells. Front Oncol., 10: 1010. doi: 10.3389/fonc.2020.01010. |
| [351] | Yamada A, Oguchi K, Fukushima M, Imai Y, Kadoya M. (2006). Evaluation of 2-Deoxy-2-[18F]fluoro-D-Glucose Positron Emission Tomography in Gastric Carcinoma: Relation to Histological Subtypes, Depth of Tumor Invasion, and Glucose Transporter-1 Expression. Ann Nucl Med., 20(9): 597–604. doi: 10.1007/BF02984657. |
| [352] | Yamaguchi H, Kitayama J, Ishigami H, Emoto S, Yamashita H, Watanabe T. (2013). A phase 2 trial of intravenous and intraperitoneal paclitaxel combined with S-1 for treatment of gastric cancer with macroscopic peritoneal metastasis. Cancer, 119: 3354–8. doi: 10.1002/cncr.28204. |
| [353] | Yamaguchi K, Yoshida K, Tanahashi T, Takahashi T, Matsuhashi N, Tanaka Y, et al. (2018). The long-term survival of stage IV gastric cancer patients with conversion therapy. Gastric Cancer, 21: 315–23. doi: 10.1007/s10120-017-0738-1. |
| [354] | Yang H, Villani RM, Wang H, Simpson MJ, Roberts MS, Tang M, et al. (2018). The Role of Cellular Reactive Oxygen Species in Cancer Chemotherapy. J Exp Clin Cancer Res., 37(1):266. doi: 10.1186/s13046-018-0909-x. |
| [355] | Yang L, Levi E, Zhu S, Du J, Majumdar AP. (2013). Cancer Stem Cells Biomarkers in Gastric Carcinogenesis. J Gastrointest Cancer, 44(4): 428–35. doi: 10.1007/s12029-013-9534-2. |
| [356] | Yang SW, Zhang ZG, Hao YX, Zhao YL, Qian F, Shi Y, et al. (2017). HIF-1alpha Induces the Epithelial-Mesenchymal Transition in Gastric Cancer Stem Cells Through the Snail Pathway. Oncotarget., 8(6): 9535–45. doi: 10.18632/oncotarget.14484. |
| [357] | Yang T, Shu X, Zhang HW, Sun LX, Yu L, Liu J, et al. (2020). Enolase 1 Regulates Stem Cell-Like Properties in Gastric Cancer Cells by Stimulating Glycolysis. Cell Death Dis., 11(10): 870. doi: 10.1038/s41419-020-03087-4. |
| [358] | Yano M, Shiozaki H, Inoue M, Tamura S, Doki Y, Yasuda T, et al. (2002). Neoadjuvant chemotherapy followed by salvage surgery: effect on survival of patients with primary noncurative gastric cancer. World J Surg., 26:1155–9. doi: 10.1007/s00268-002-6362-0. |
| [359] | Yao S, Shang W, Huang L, Xu R, Wu M, Wang F. (2021). The Oncogenic and Prognostic Role of PDK1 in the Progression and Metastasis of Ovarian Cancer. J Cancer, 12(3):630–43. doi: 10.7150/jca.47278. |
| [360] | Yasumoto Y, Miyazaki H, Vaidyan LK, Kagawa Y, Ebrahimi M, Yamamoto Y, et al. (2016). Inhibition of Fatty Acid Synthase Decreases Expression of Stemness Markers in Glioma Stem Cells. PloS One, 11(1): e0147717. doi: 10.1371/journal.pone.0147717. |
| [361] | Ye J, Huang Q, Xu J, Huang J, Wang J, Zhong W, et al. (2018). Targeting of Glutamine Transporter ASCT2 and Glutamine Synthetase Suppresses Gastric Cancer Cell Growth. J Cancer Res Clin Oncol., 144(5): 821–33. doi: 10.1007/s00432-018-2605-9. |
| [362] | Yin J, Wu X, Li S, Li C, Guo Z. (2020). Impact of Environmental Factors on Gastric Cancer: A Review of the Scientific Evidence, Human Prevention and Adaptation. J Environ Sci., 89:65–79. doi: 10.1016/j.jes.2019.09.025. |
| [363] | Yokoyama A, Omori T. (2005). Genetic polymorphisms of alcohol and aldehyde dehydrogenases and risk for esophageal and head and neck cancersExit Disclaimer. Alcohol, 35(3): 175-185. |
| [364] | Yoshida K, Yamaguchi K, Okumura N, Tanahashi T, Kodera Y. (2016). Is conversion therapy possible in stage IV gastric cancer: the proposal of new biological categories of classification. Gastric Cancer, 19: 329–38. doi: 10.1007/s10120-015-0575-z. |
| [365] | Yoshida M, Ohtsu A, Boku N, Miyata Y, Shirao K, Shimada Y, et al. (2004). Long-term survival and prognostic factors in patients with metastatic gastric cancers treated with chemotherapy in the Japan Clinical Oncology Group (JCOG) study. Jpn J Clin Oncol., 34: 654–9. doi: 10.1093/jjco/hyh120. |
| [366] | Yoshikawa T, Sasako M, Yamamoto S, Sano T, Imamura H, Fujitani K, et al. (2009). Phase II study of neoadjuvant chemotherapy and extended surgery for locally advanced gastric cancer. Br J Surg., 96: 1015–22. doi: 10.1002/bjs.6665. |
| [367] | Yoshikawa T, Tanabe K, Nishikawa K, Ito Y, Matsui T, Kimura Y, et al. (2014). Induction of a pathological complete response by four courses of neoadjuvant chemotherapy for gastric cancer: early results of the randomized phase II COMPASS trial. Ann Surg Oncol., 21: 213–9. doi: 10.1245/s10434-013-3055-x. |
| [368] | Yu Z, Pestell TG, Lisanti MP, Pestell RG. (2012). Cancer Stem Cells. Int J Biochem Cell Biol., 44(12): 2144–51. doi: 10.1016/j.biocel.2012.08.022. |
| [369] | Yuan LW, Yamashita H, Seto Y. (2016). Glucose Metabolism in Gastric Cancer: The Cutting-Edge. World J Gastroenterol., 22(6): 2046–59. doi: 10.3748/wjg.v22.i6.2046. |
| [370] | Zaidieh T, Smith JR, Ball KE. (2019). An Q. ROS as a Novel Indicator to Predict Anticancer Drug Efficacy. BMC Cancer, 19(1): 1224. doi: 10.1186/s12885-019-6438-y. |
| [371] | Zavros Y. (2017). Initiation and Maintenance of Gastric Cancer: A Focus on CD44Variant Isoforms and Cancer Stem Cells. Cell Mol Gastroenterol Hepatol., 4(1): 55–63. doi: 10.1016/j.jcmgh.2017.03.003. |
| [372] | Zhang J, Pavlova NN, Thompson CB. (2017). Cancer Cell Metabolism: The Essential Role of the Nonessential Amino Acid, Glutamine. EMBO J., 36 (10): 1302–15. doi: 10.15252/embj.201696151. |
| [373] | Zhang Q, Han Z, Zhu Y, Chen J, Li W. (2021). Role of Hypoxia Inducible Factor-1 in Cancer Stem Cells (Review). Mol Med Rep., 23(1): 1. doi: 10.3892/mmr.2020.11655. |
| [374] | Zhao J, Stockwell T, Roemer A, Chikritzhs T. (2016). Is alcohol consumption a risk factor for prostate cancer? A systematic review and meta-analysis. BMC Cancer, 16(1): 845. |
| [375] | Zhao M, Hou Y, Du YE, Yang L, Qin Y, Peng M, et al. (2020). Drosha-Independent miR-6778-5p Strengthens Gastric Cancer Stem Cell Stemness via Regulation of Cytosolic One-Carbon Folate Metabolism. Cancer Lett., 478: 8–21. doi: 10.1016/j.canlet.2020.02.040. |
| [376] | Zhao W, Li Y, Zhang X. (2017). Stemness-Related Markers in Cancer. Cancer Transl Med., 3(3): 87–95. doi: 10.4103/ctm.ctm_69_16. |
| [377] | Zhou D, Jiang L, Jin L, Yao Y, Wang P, Zhu X. (2020). Glucose Transporter-1 Cooperating With AKT Signaling Promote Gastric Cancer Progression. Cancer Manag Res., 12:4151–60. doi: 10.2147/CMAR.S251596. |
| [378] | Zhou D, Shao L, Spitz DR. (2014). Reactive Oxygen Species in Normal and Tumor Stem Cells. Adv Cancer Res., 122: 1–67. doi: 10.1016/B978-0-12-420117-0.00001-3. |
| [379] | Zhou HM, Zhang JG, Zhang X, Li Q. (2021) Targeting Cancer Stem Cells for Reversing Therapy Resistance: Mechanism, Signaling, and Prospective Agents. Signal Transduct Target Ther., 6(1): 62. doi: 10.1038/s41392-020-00430-1. |
| [380] | Zhou, T., Yang Y, Chen Q, Xie L. (2019). Glutamine Metabolism Is Essential for Stemness of Bone Marrow Mesenchymal Stem Cells and Bone Homeostasis. Stem Cells Int., 2019: 8928934. doi: 10.1155/2019/8928934. |
| [381] | Zhu, X., Chen HH, Gao CY, Zhang XX, Jiang JX, Zhang Y, et al. (2020). Energy Metabolism in Cancer Stem Cells. World J Stem Cells, 12(6): 448–61. doi: 10.4252/wjsc.v12.i6.448. |
| [382] | Zurleni, T., Gjoni E, Altomare M, Rausei S. (2018). Conversion surgery for gastric cancer patients: a review. World J Gastrointestinal Oncol., 10: 398–409. doi: 10.4251/wjgo.v10.i11.398. |
| [383] | Young, J. J., Pahwa, A., Patel, M., Jude, C. M., Nguyen, M., Deshmukh, M., … Mohammad, S. F. (2019). Ligaments and Lymphatic Pathways in Gastric Adenocarcinoma. RadioGraphics, 180113. doi:10.1148/rg.2019180113. |
| [384] | Ku, C.C., Wuputra, K., Pan, J.B., Li, C.P., Liu, C.J., Liu, Y.C., Saito, S., Chan, T.F., Lin, C.S., Wu, D.C. & Yokoyama, K.K. (2022). Generation of Human Stomach Cancer iPSC-Derived Organoids Induced by Helicobacter pylori Infection and Their Application to Gastric Cancer Research. Cells, 11, 184. https://doi.org/10.3390/cells11020184. |
| [385] | Barker, N.; Huch, M.; Kujala, P.; van de Wetering, M.; Snippert, H.J.; van Es, J.H.; Sato, T.; Stange, D.E.; Begthel, H.;van den Born, M. ; et al. Lgr5+ve Stem Cells Drive Self-Renewal in the Stomach and Build Long-Lived Gastric Units In Vitro.Cell Stem Cell 2010, 6, 25–36. [CrossRef] [PubMed] |
| [386] | DeWard, A.D.; Cramer, J.; Lagasse, E. Cellular heterogeneity in the mouse esophagus implicates the presence of a nonquiescent epithelial stem cell population. Cell Rep. 2014, 9, 701–711. |
| [387] | Katano, T.; Ootani, A.; Mizoshita, T.; Tanida, S.; Tsukamoto, H.; Ozeki, K.; Ebi, M.; Mori, Y.; Kataoka, H.; Kamiya, T.; et al. Establishment of a long-term three-dimensional primary culture of mouse glandular stomach epithelial cells within the stem cell niche. Biochem. Biophys. Res. Commun. 2013, 432, 558–563. [CrossRef] [PubMed] |
| [388] | Miyoshi, H.; Stappenbeck, T.S. In vitro expansion and genetic modification of gastrointestinal stem cells in spheroid culture. Nat. Protoc. 2013, 8, 2471–2482. [CrossRef] [PubMed] |
| [389] | Li, X.; Nadauld, L.; Ootani, A.; Corney, D.C.; Pai, R.K.; Gevaert, O.; Cantrell, M.A.; Rack, P.G.; Neal, J.T.; Chan, C.W.; et al. Oncogenic transformation of diverse gastrointestinal tissues in primary organoid culture. Nat. Med. 2014, 20, 769–777. [CrossRef] |
| [390] | McCracken, K.W.; Catá, E.M.; Crawford, C.M.; Sinagoga, K.L.; Schumacher, M.; Rockich, B.E.; Tsai, Y.-H.; Mayhew, C.N.; Spence, J.R.; Zavros, Y.; et al. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 2014, 516, 400–404. [CrossRef] [PubMed] |
| [391] | Hannan, N.R.; Fordham, R.P.; Syed, Y.A.; Moignard, V.; Berry, A.; Bautista, R.; Hanley, N.A.; Jensen, K.B.; Vallier, L. Generation of multipotent foregut stem cells from human pluripotent stem cells. Stem Cell Rep. 2013, 1, 293–306. [CrossRef] [PubMed] |
| [392] | Zhang, F., Huang, X., Song, Y., Gao, P., Zhou, C., Guo, Z., Shi, J., Wu, Z. and Wang, Z. (2019). Conversion Surgery for Stage IV Gastric Cancer. Frontiers in Oncology, 9: 1158, 1-10. doi: 10.3389/fonc.2019.01158. |
| [393] | Kim SW. (2014). The result of conversion surgery in gastric cancer patients with peritoneal seeding. J Gastr Cancer, 14: 266–70. doi: 10.5230/jgc.2014.14.4.266. |
| [394] | Chan DY, Syn NL, Yap R, Phua JN, Soh TI, Chee CE, et al. (2017). Conversion surgery post-intraperitoneal paclitaxel and systemic chemotherapy for gastric cancer carcinomatosis peritonei. are we ready? J. Gastrointest Surg., 21:425–33. doi: 10.1007/s11605-016-3336-3. |
| [395] | Thomassen I, van Gestel YR, van Ramshorst B, Luyer MD, Bosscha K, Nienhuijs SW, et al. (2014). Peritoneal carcinomatosis of gastric origin: a population-based study on incidence, survival and risk factors. Int J Cancer, 134: 622–8. doi: 10.1002/ijc.28373. |
| [396] | Lemaire, M. & Halperin, M.L. (2009). Rapid tumor cell swelling and bursting: beware of collateral damage. Molecular Therapy, 17(8): 1310-1311. doi: 10.1038/mt.2009.161. PMID: 19644494; PMCID: PMC2835251. |
| [397] | Namikawa, T., Munekage, E., Ogawa, M., Oki, T., Munekage, M., Maeda, H., Kitagawa, H., Sugimoto, T., Kobayashi, M. and Hanazaki, K. (2017). Clinical presentation and treatment of gastric metastasis from other malignancies of solid organs. Biomedical Reports, 7(2), 159–162. doi:10.3892/br.2017.943. |
| [398] | Namikawa T and Hanazaki K (2014). Clinicopathological features and treatment outcomes of metastatic tumors in the stomach. Surgery Today, 44: 1392-1399. doi: https://doi.org/10.1007/s00595-013-0671-9. |
| [399] | Oda, I., Kondo, H., Yamao, T., Saito, D., Ono, H., Gotoda, T., Yamaguchi, H., Yoshida, S. and Shimoda, T. (2001). Metastatic tumors to the stomach: Analysis of 54 patients diagnosed at endoscopy and 347 autopsy cases. Endoscopy, 33: 507-510. doi: 10.1055/s-2001-14960, PMID: 11437044. |
| [400] | Kobayashi O, Murakami H, Yoshida T, Cho H, Yoshikawa T, Tsuburaya A, Sairenji M, Motohashi H, Sugiyama Y and Kameda Y. (2004). Clinical diagnosis of metastatic gastric tumors: Clinicopathologic findings and prognosis of nine patients in a single cancer center. World Journal of Surgery, 28: 548-551. doi: https://doi.org/10.1007/s00268-004-7216-8. |
| [401] | Uddin, M.B., Hoque, M., Ali, M.M., Islam, A. and Miah, M.R. (2021). Abandonment and Outcome of Induction Chemotherapy in Childhood Acute Lymphoblastic Leukemia. Research In Cancer and Tumor, 9(1), 8-14. doi: 10.5923/j.rct.20210901.02. url: http://article.sapub.org/10.5923.j.rct.20210901.02.html. |
| [402] | Hossain, M.S., Miah, M.R., Fardous, M., Ferdous, N.J., Mostofa, M.G., Hossain, S.A.M.I., Shahriar, C.S., Talukdar, M.T.H. and Ansari, M.A.S. (2021). Histopathological Study of Oral and Oropharyngeal Lesions in a Tertiary Care Hospital. Research In Cancer and Tumor, 9(1), 1-7. doi: 10.5923/j.rct.20210901.01. url: http://article.sapub.org/10.5923.j.rct.20210901.01.html. |
| [403] | Bonilla, M., Rossell, N., Salaverria, C., Gupta, S., Barr, R., Sala, A., Metzger, M.L. and Sung, L. (2009). Prevalence and Predictors of Abandonment of Therapy Among Children with Cancer in EL Salvador. International Journal of Cancer, 125(9): 2144-2146. doi: 10.1002/ijc.24534. PMID: 19585496. |



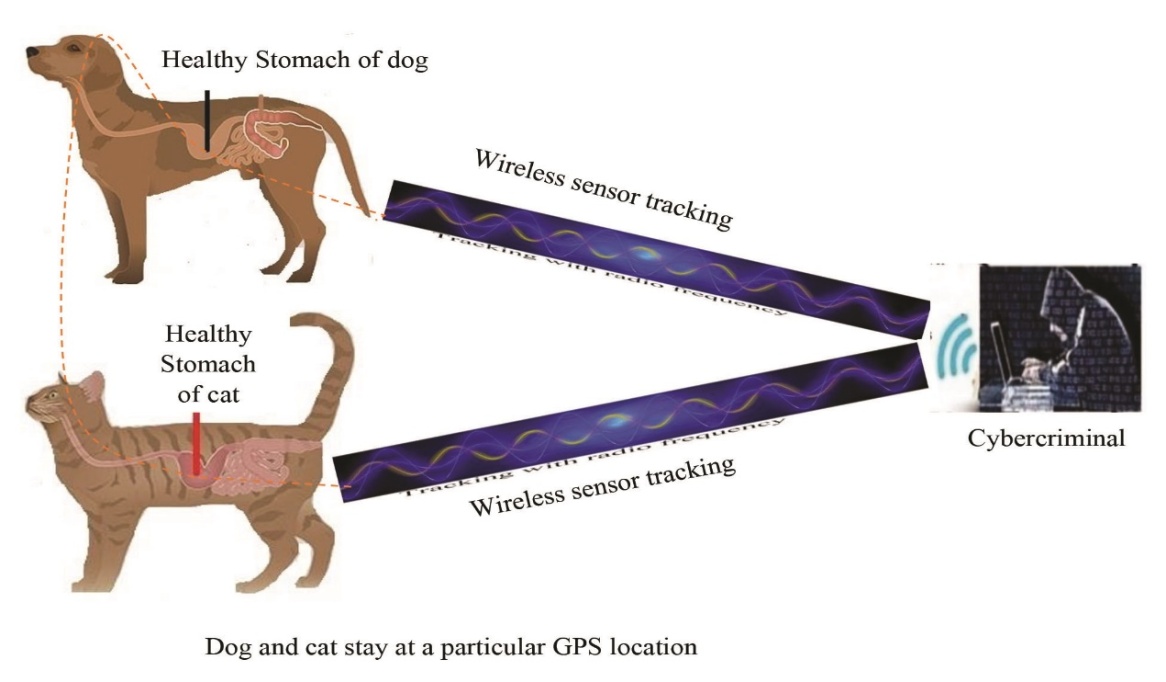
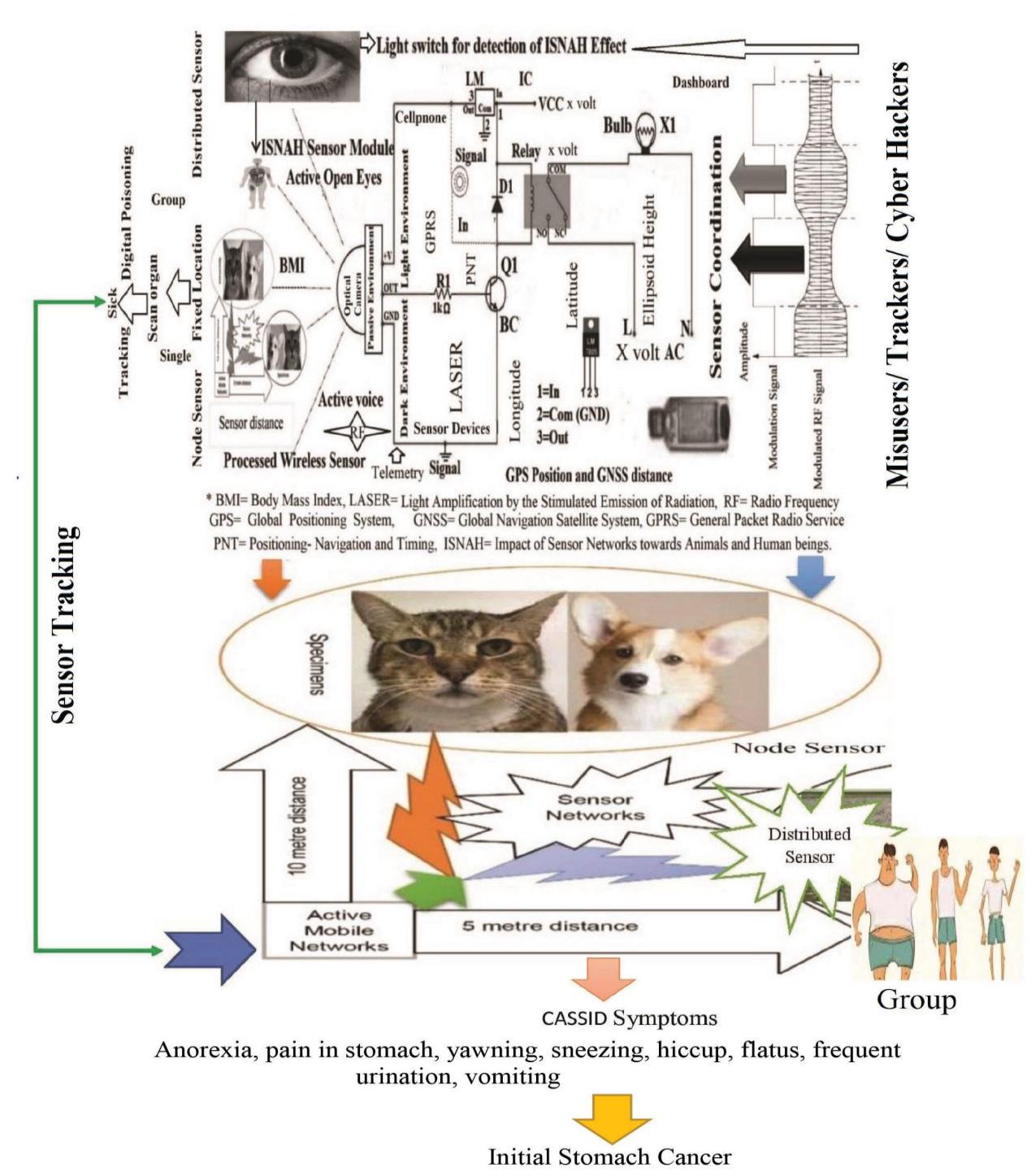
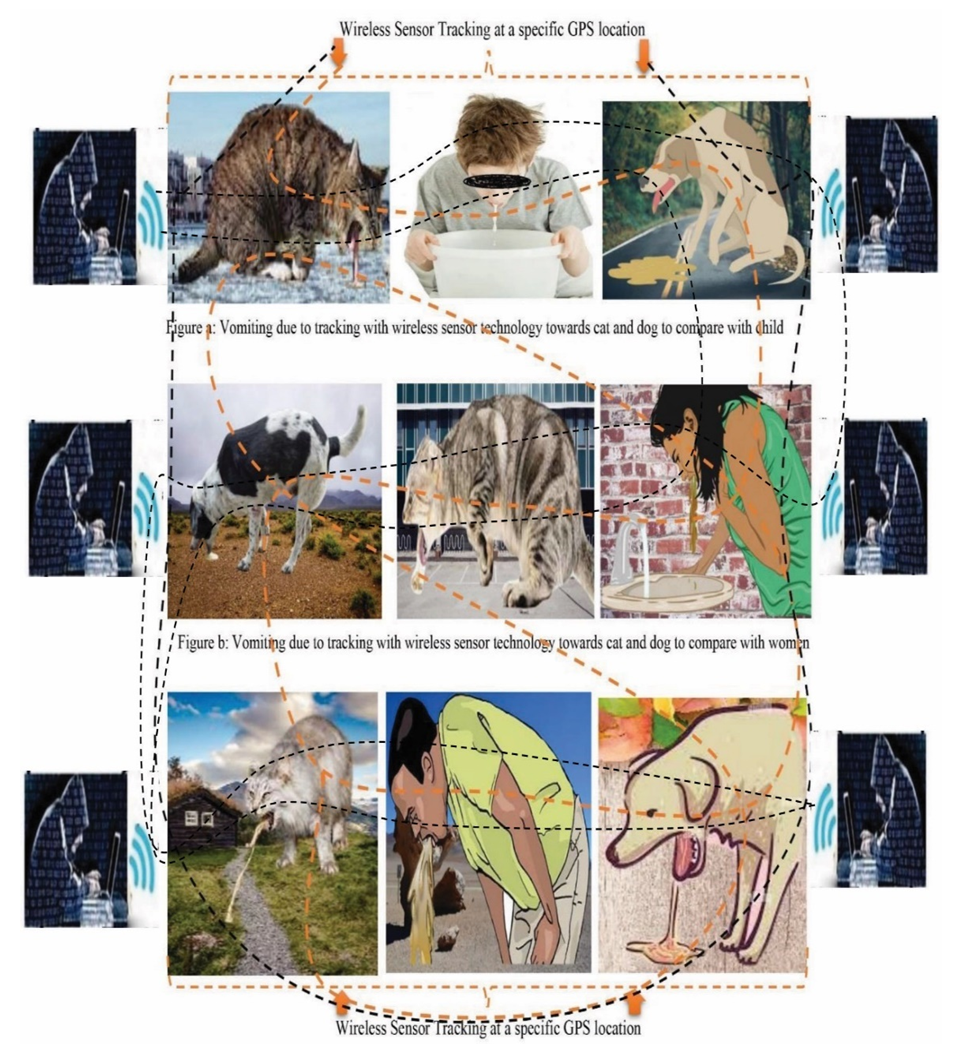

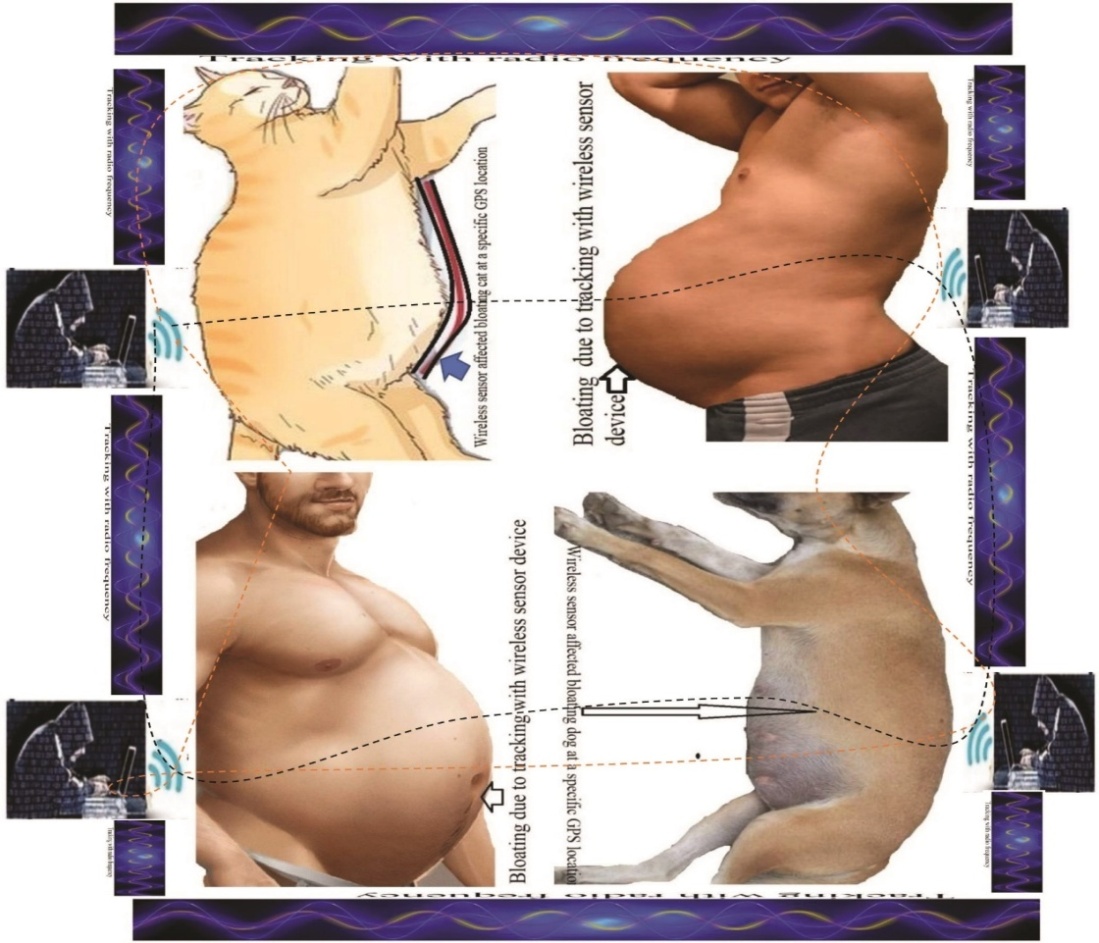

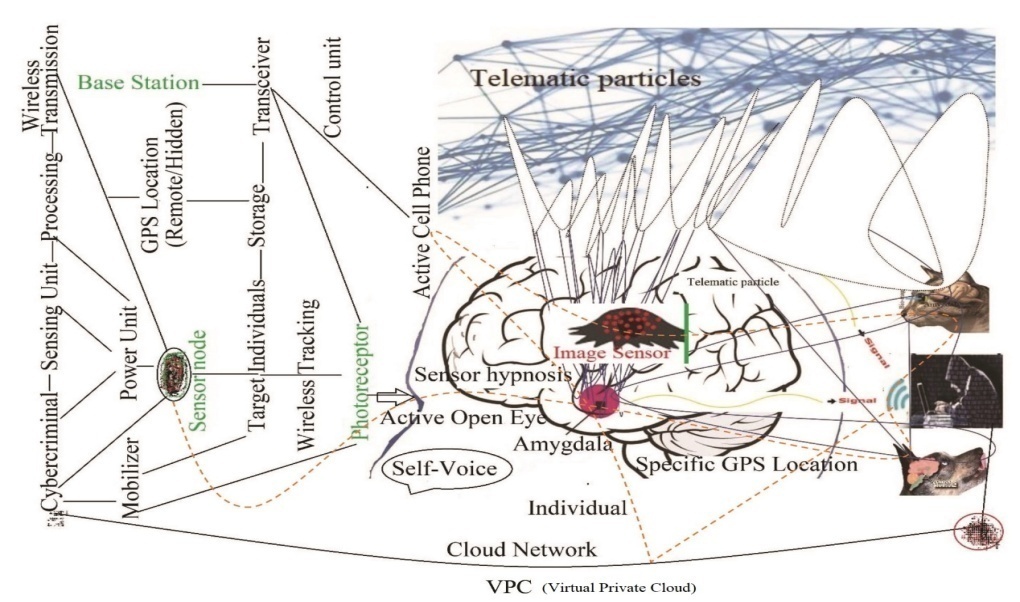
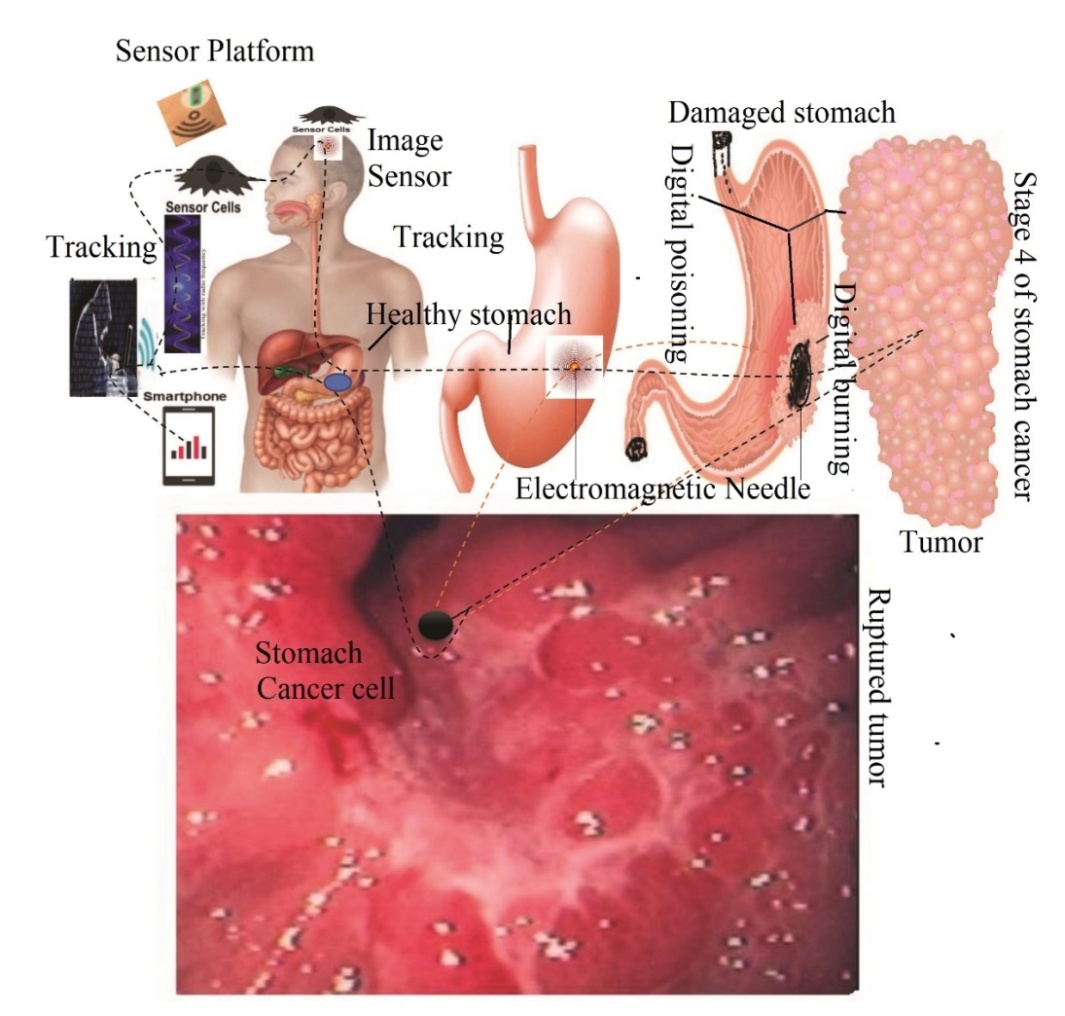
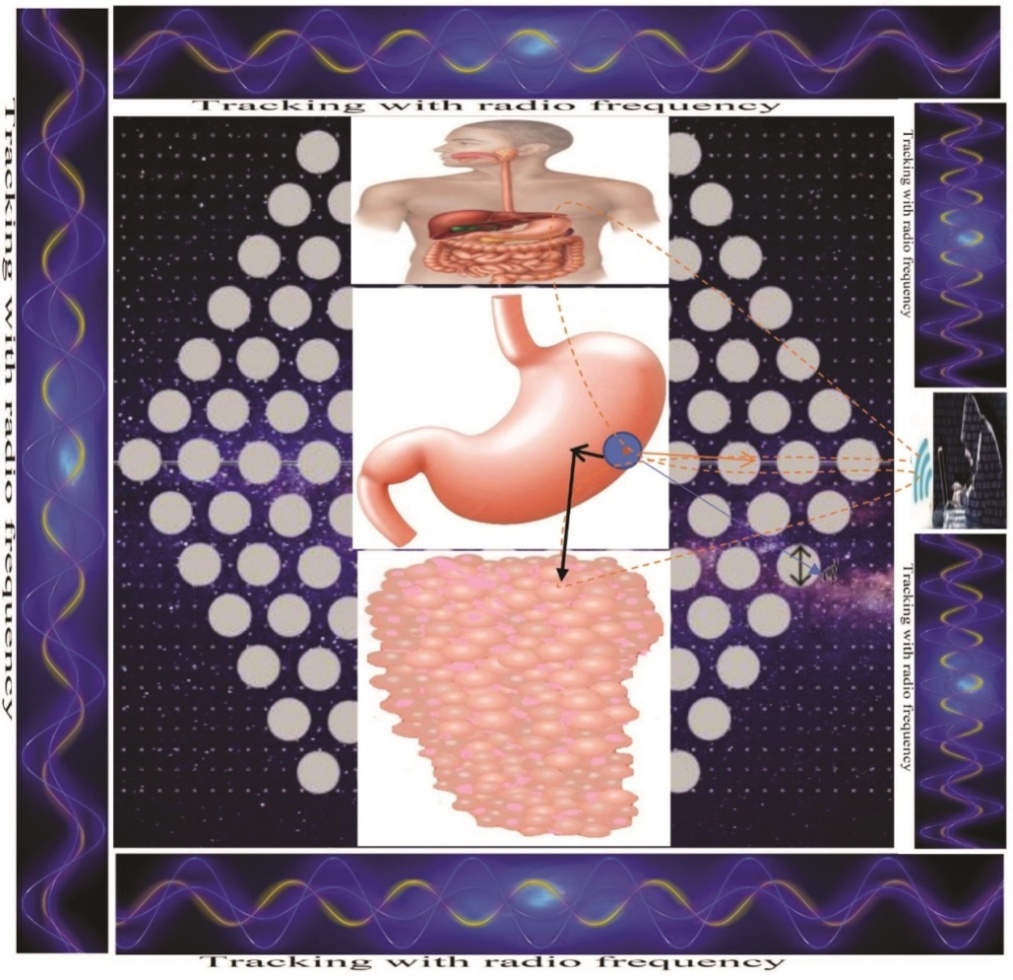
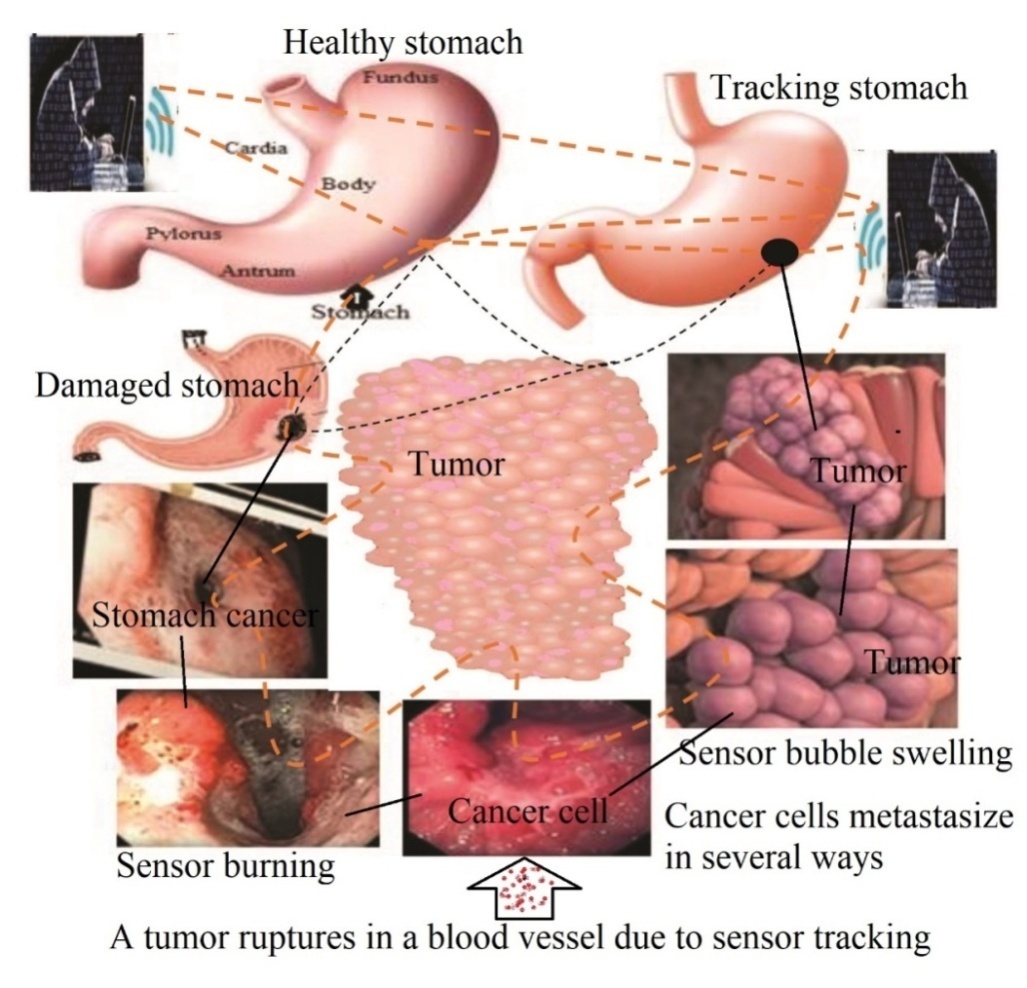
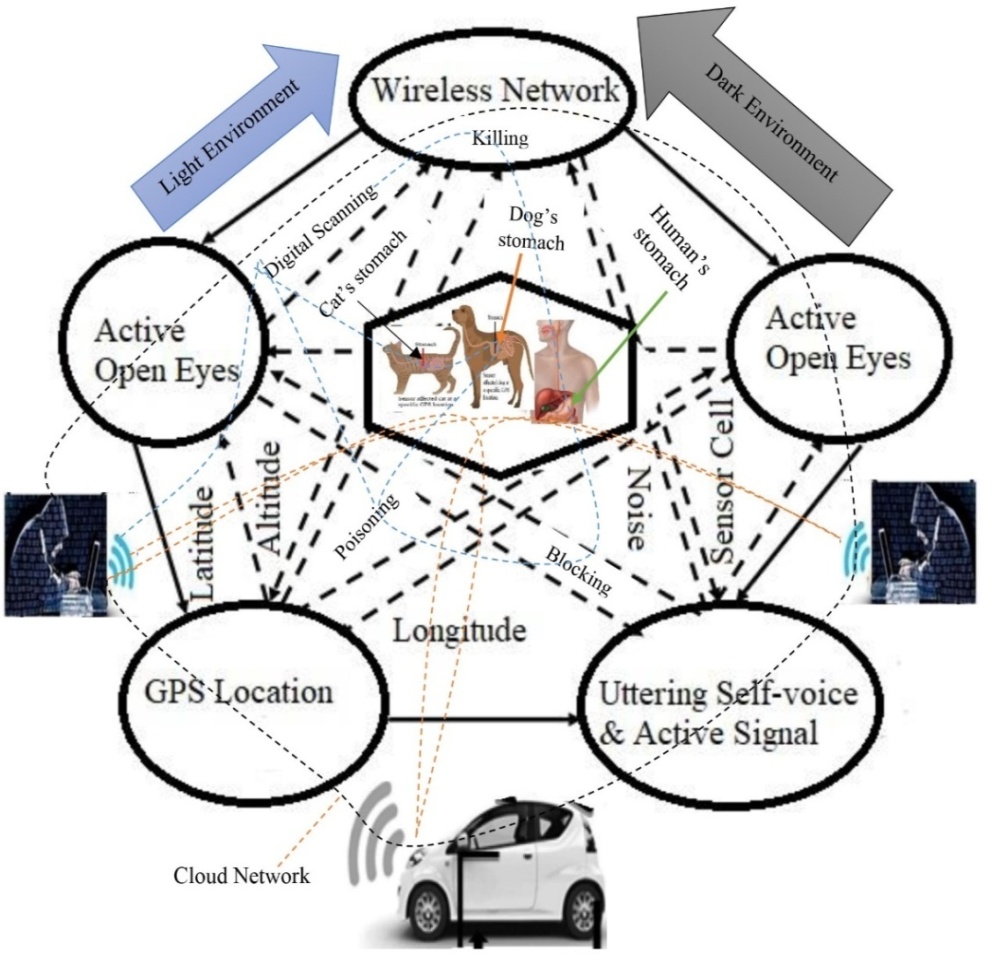
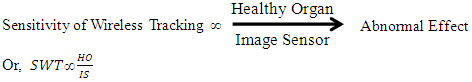 For example, Sensitivity of Wireless Tracking ∞Healthy Stomach/Image Sensor.Overall, the study represents on the associated risk factors of stomach cancer due to uncontrolled wireless tracking at a particular GPS location. So, its recovery system with suitable manner is urgent.
For example, Sensitivity of Wireless Tracking ∞Healthy Stomach/Image Sensor.Overall, the study represents on the associated risk factors of stomach cancer due to uncontrolled wireless tracking at a particular GPS location. So, its recovery system with suitable manner is urgent.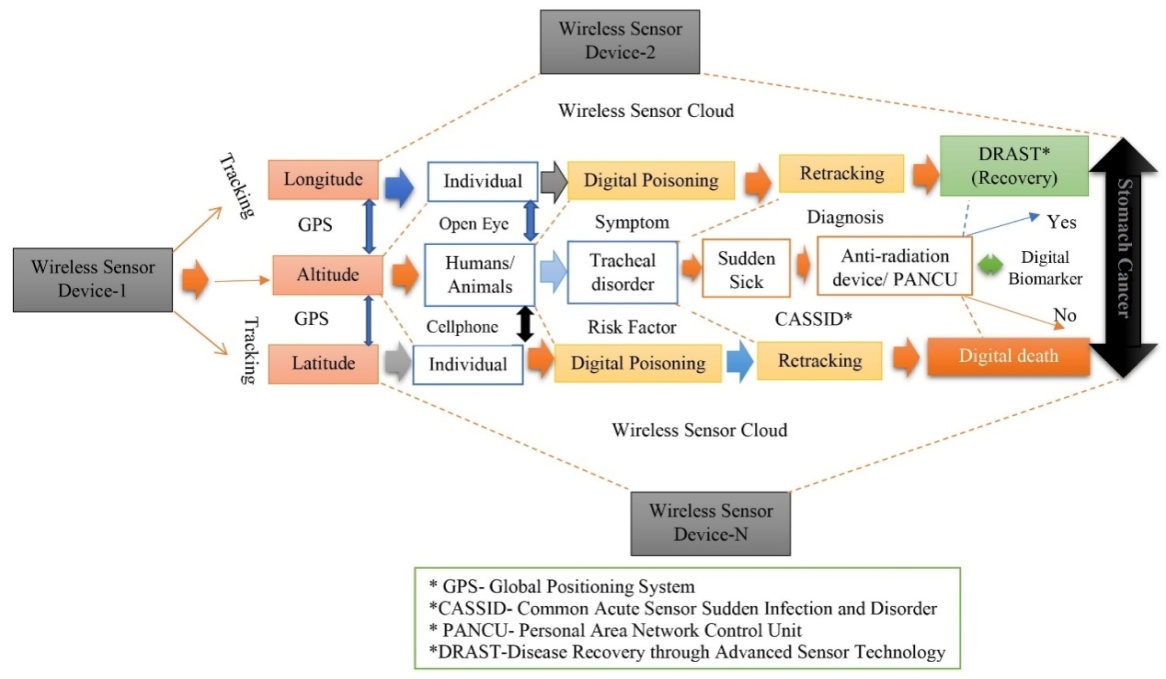
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML