-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Stem Cell Research
p-ISSN: 2325-0097 e-ISSN: 2325-0089
2023; 5(1): 1-7
doi:10.5923/j.ajscr.20230501.01
Received: Dec. 11, 2022; Accepted: Jan. 6, 2023; Published: Jan. 13, 2023

Suprachoroidal Umbilical Cord Derived Mesenchymal Stem Cell Implantation for the Treatment of Retinitis Pigmentosa in Pediatric Patients
Ayse Oner1, Neslihan Sinim Kahraman2
1Acibadem Health Group, Acibadem Taksim Hospital, Istanbul, Turkey
2Acibadem University, Acibadem Taksim Hospital, Istanbul, Turkey
Correspondence to: Ayse Oner, Acibadem Health Group, Acibadem Taksim Hospital, Istanbul, Turkey.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: This study shows the clinical data of pediatric patients with retinitis pigmentosa (RP) who received suprachoroidal umbilical cord derived mesenchymal stem cell (UCMSC) implantation. Methods: This prospective, single-center, study enrolled 46 eyes of 46 pediatric patients (age 18 years or younger) with RP who underwent suprachoroidal implantation of UCMSCs. All patients had varying degrees of visual field (VF) defects and visual loss and defective multifocal electroretinography (mf ERG). Patients were evaluated on the first day, first month, sixth month and at the end of the first year postoperatively. Best corrected visual acuity (BCVA), anterior segment and fundus examination, color photography, optical coherence tomography (OCT) and VF examination were carried out at each visit. Fundus fluorescein angiography (FFA) and mfERG recordings were performed at baseline and at the end of the sixth month and first year. Results: All 46 patients completed 12-month follow-up period. None of them had any systemic or ocular complications. We found statistically significant improvements in visual acuity, VF and mfERG recordings. Conclusions: Even though the sample size is small, stem cell treatment with suprachoroidal implantation of UCMSCs seems to be safe and the improvements were encouraging. To optimize the cell delivery technique and to evaluate the effects of this therapy on visual acuity and the quality of life of pediatric patients, future studies with larger number of cases with longer follow-up periods will be necessary.
Keywords: Umbilical cord derived mesenchymal stem cell, Pediatric patient, Retinitis pigmentosa, Suprachoroidal Implantation
Cite this paper: Ayse Oner, Neslihan Sinim Kahraman, Suprachoroidal Umbilical Cord Derived Mesenchymal Stem Cell Implantation for the Treatment of Retinitis Pigmentosa in Pediatric Patients, American Journal of Stem Cell Research, Vol. 5 No. 1, 2023, pp. 1-7. doi: 10.5923/j.ajscr.20230501.01.
1. Introduction
- Inherited retinal disorders (IRDs) are an important cause of visual impairment in the pediatric population. They include a large group of pathology which are heterogeneous both clinically and genetically. Retinitis pigmentosa (RP) is the most common form of IRDs which is characterized by progressive nyctalopia, constricting visual fields, followed by severe visual loss and even blindness by the third or fourth decade of life. The disease is initially caused by rod degeneration eventually affecting the cones. It has a worldwide prevalence of 1 in 4000. Clinical findings of fundus examination include attenuation of the vessels, optic disc pallor and bone-spicule intraretinal pigment migration. [1-3]Although there is no curative treatment for RP up to date, new approaches including restoring defective genes and stem cell transplantation to replace dead cells or to restore defective cells have been under investigation. [2] Stem cell treatment holds great promise, as the same therapeutic agent can be used irrespective of the underlying etiology. Mesenchymal stem cells (MSCs) are currently being investigated extensively for the treatment of degenerative retinal diseases. [2-6] These stem cells are proved to have anti-apoptotic, anti-inflammatory, immunomodulatory and angiogenic effects. They also provide trophic support for the neuroprotection and regeneration of damaged retinal cells either directly through the secretion of neurotrophic factors (NTFs) or indirectly with the paracrine support. [2-6] Previous experimental data demonstrated the beneficial therapeutic effects of MSCs on retinal diseases. It has been shown that MSC transplantation significantly delays retinal degeneration, supports the regeneration, and improves the survival of retinal cells. [5,6] The application of MSCs in clinical studies also appears to be safe and no serious treatment-related problems were observed in the treated patients with retinal diseases. [3,4,6-9] Recent clinical studies including umbilical cord-derived MSC (UCMSC) application have reported significant paracrine and immunomodulatory features by producing trophic factors and this treatment seems to be effective in preventing retinal degeneration and support the survival of the residual retinal cells. These studies suggested that growth factors secreted by the UCMSCs may lead to reactivation of the photoreceptors in the dormant phase and the regeneration of synaptic connections. A significant increase in the outer retinal thickness and improvement in visual functions found in the studies can be explained through this mechanism. The authors also claimed that the photoreceptors which have undergone apoptosis would not respond to UCMSC implantation therefore this therapy will be more beneficial if it is performed in early stages of the disease while there are more live photoreceptors. [3,4,9]Previous clinical studies of stem cell implantation included patients older than 18 years and showed no serious side effects. Considering the progressive nature of the disease and the fact that MSC application would not regenerate the apopitotic photoreceptors and also with the critical evidence of safe results of MSC application in adults; the authors believe that clinical trials need to be conducted in pediatric patients to get better results. Here we aim to report the medium term -1 year- results of pediatric patients with RP who received suprachoroidal UCMSC implantation and to discuss the safety and tolerability of the procedure in children. To the best of our knowledge this is the first report including the application of stem cells in retinal diseases including pediatric age group.
2. Patients and Methods
- This single-center, prospective, clinical safety study included 46 eyes of 46 pediatric patients with decreased vision from RP seen in the Retina and Vitreous Section of the Department of Ophthalmology at our Health Group. The study was performed in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board (Review number for approval (2017/480, 10.13.2017) It was also approved by the Review Board of Stem Cell Applications within the Ministry of Health in accordance with the regulations in our country (Review number for approval: 56733164/203). All patients and parents were instructed about the objectives and methodology of the study and gave their written informed consent to participate.Patient eligibility: There is no previous data about clinical applications of MSCs in pediatric patients and the safety of this therapy still needs to be clarified. Therefore, the study included patients with low vision. Patients were included if they had: 1) A clinical and ophthamological diagnosis of RP 2) Snellen best corrected visual acuity (BCVA) worse than 20/50 (0.4 Snellen lines) 3) Visual field defects 4) Decreased flash and multifocal electroretinography (ERG) recordings 5) Age younger than 18 years 6) Compliance for visual field and ERG tests.Exclusion criteria were: 1) Previous ocular surgery other than cataract extraction 2) Presence of cataract or other media opacity that would affect high-quality ocular imaging, ERG or visual field evaluation 3) Presence of other ophthalmic disease, such as glaucoma, uveitis, strabismus or nystagmus 4) Any other systemic disease such as neurological disease that would affect the results. If both eyes were eligible for treatment, the eye with worse visual acuity was operated. The demographic data of the patients were collected, ophthalmic evaluations and surgical procedures were performed by a single retina specialist (A.O). Fundus photography, optical coherence tomography (OCT), perimetry, flash and multifocal ERG recordings were performed by the same experienced ophthalmic technician. The patients completed perimetry and multifocal ERG twice on different days before the baseline evaluation to enhance the familiarity to the tests. Perimetry and electrophysiological tests were evaluated by a masked researcher (NSK).All patients had a complete ophthalmic examination before surgery. BCVA was measured with Snellen chart. All patients underwent fundus photography using the Zeiss FF 450 (Carl Zeiss Meditec AG, Germany). OCT was performed using the Nidek RS- 3000 OCT Device (Nidek CO Ltd. Japan) with a standardized scanning protocol. Visual field examination was performed by Humphrey VF analyzer device (Carl Zeiss Meditec AG Germany), program 30-2 was used for testing of each eye. ERG (Monpack 3, Metrovision, France) readings were recorded from each eye according to the International Society for Clinical Electrophysiology of Vision (ISCEV) guidelines. [10]The stem cell preparation and surgical procedure:Stem Cell Preparation: Umbilical cord tissue is used to obtain MSCs. A single umbilical cord contains 2-3 bilion and 1 centimeter area of the tissue contains 3-5 million MSCs. The production protocol of UC-MSCs was previously described elsewhere. [3] After extraction from the cord tissue, the cells were expanded ex vivo to reach the desired number of cells. To maintain the genetic and phenotypic properties, the cells were not passaged more than two times. The preparation procedure was performed under good manufacturing practice (GMP) conditions and before release UCMSCs were assessed for cell appearance, viability, identification, purity, content, and potency. The cells were also screened for bacterial or fungal contamination. Cell viability was>90.0%±0.5% before cell transplantation. A concentration of 5 milion cells in isotonic solution containing 1% human serum albumin were carried in 2 mL vials which was placed in a sterile tube. The tubes were transfered with the temperature-controlled bag (set at a temperature between 2°C-8°C) until the cells reach the operating room. The cells were used within 24h.Surgical Procedure: We performed a surgical technique defined as the Limoli retinal restoration technique (LRRT), described by Limoli et al. [8] An experienced surgeon (A.O) performed all surgeries. All operations were done under general anesthesia. The details of the surgery were as follows: The globe was deviated to the supero-nasal quadrant and conjunctiva was dissected at the infero-temporal quadrant at 8 mm from the limbus. A deep scleral flap of about 5×5 mm was opened by radial hinge at the infero-temporal quadrant. The sclerectomy was deep enough to allow viewing of the color of the choroid. A flap from the orbital fat was extracted from a gap above the inferior oblique muscle. This tissue was laid on the scleral bed and sutured with 6/0 vicryl at the proximal edge. The scleral flap was then sutured above the fat pedicle. The remaining space between the autologous fat graft, choroid, and scleral flap was filled with 1 cc of 5×106 UC-MSCs using a 25-gauge cannula. The conjunctiva was sutured with 8/0 vicryl.Patients were recommended to use topical antibiotic and steroid eye drops four times a day for 1month after surgery. Routine ophthalmic evaluation, color fundus photograph, visual field and OCT were performed before surgery, and at the postoperative 1st, 3rd, 6th and 12th months. We also performed mfERG before surgery and at 6th and 12th months postoperatively. All patients were evaluated for adverse events of the surgical procedures during the study period.
3. Results
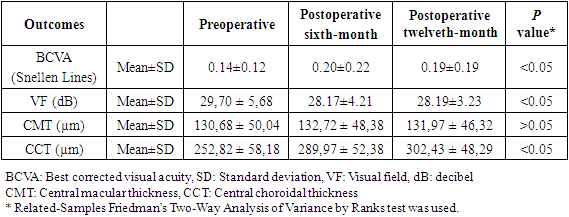
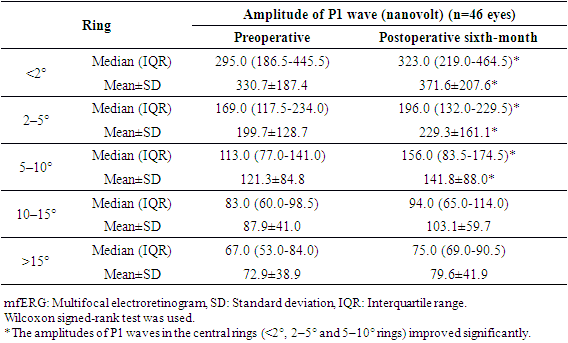
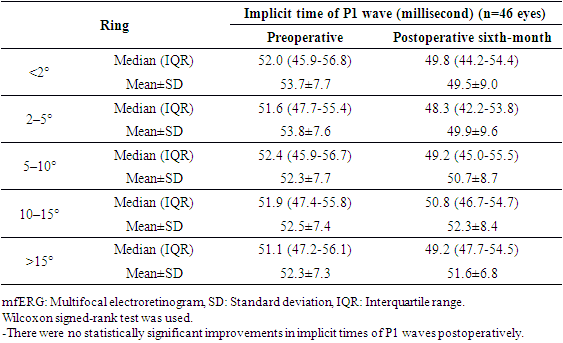
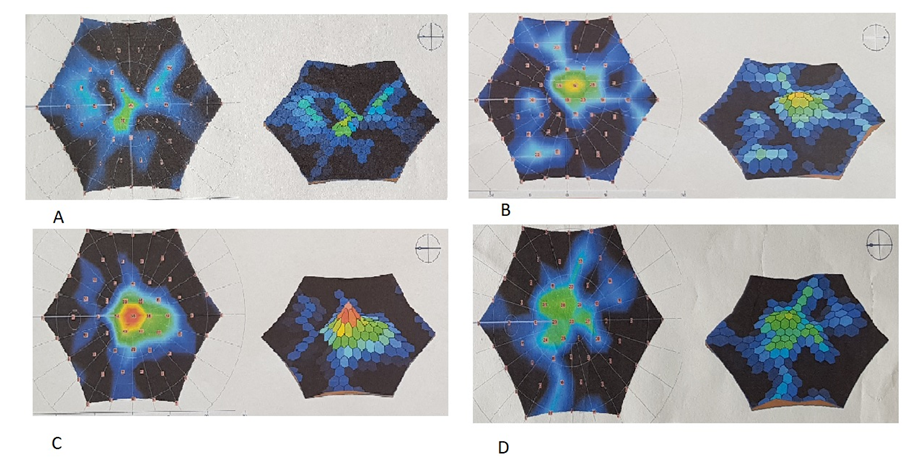
- Table 1 shows the demographic and clinical features of the 46 study patients who enrolled in this study. The age of the subjects ranged between 9 and 17 years.
|
|
|
|
4. Discussion
- Retinitis pigmentosa is the most common inherited retinal dystrophy which causes a severe progressive vision loss in children. A recent publication reported that the disease causes a mean reduction of the mean deviation (MD) value of the visual field (VF) between −0.37 to -0.89 dB in a year. [11] An optical coherence tomography (OCT) study also showed a mean rate of 7% decline in the ellipsoid zone band width which was translated into a mean rate of change of 13% for the equivalent area of functioning retina. [12] Considering the progressive nature of RP, the development of therapies to slow down or to prevent the progression of the disease is required. Despite considerable efforts, the treatment possibilities for the disease remain very limited. [13] Collective data from the therapeutic options which have been studied recently including gene therapy, [14] electrical stimulation, [15] and stem cell therapy [3,4] emphasized the availability of functioning photoreceptors for better visual outcomes after interventions. Current study in pediatric patients was conducted in terms of this crucial evidence accumulated from these recent publications. Previous experimental and clinical data showed the beneficial therapeutic effects of MSC implantation on retinal diseases. An advantage of MSCs is their safety in use. An extensive meta-analysis of studies using MSCs in over 1000 patients did not reveal a significant association between MSC treatment and the toxicity of infusions, internal organ infection, cancer or death. [16] There were also no significant safety issues or severe adverse effects related to the intravenous and intrathecal injections of MSCs treatment in pediatric patients diagnosed with autism. [17] This current study also confirmed that local application of MSCs had no side effects in 1 year follow-up period in pediatric patients. There are several studies in the literature including MSC treatment of RP patients in adults. In a recent phase I/II clinical trial 32 RP patients were intravenously infused with one dose of 100 milion UCMSC and were followed up for one year. Most patients improved their BCVA in the first 3 months. Most of the patients (81.3%) maintained or improved their visual acuities for 12 months. Although the average visual field sensitivity and flash visual evoked potential showed no significant difference, the average NEI VFQ-25 questionnaire scores were significantly improved at the third month. The authors reported that the intravenous infusion of UCMSCs was safe for advanced RP patients and most of the patients improved or maintained their visual functions in the long term. [18]Suprachoroidal implantation method has been first introduced by Limoli and his colleagues and was proven to be safe with no complications in recent publications. [8,19,20] In a recent study including UCMSC implantation in patients with RP, 124 eyes of 82 patients received 5 million UCMSCs to the suprachoroidal area with Limoli technique. None of the patients had any serious systemic or ocular complications. There were statistically significant improvements in BCVA and VF during the study. The authors also reported significant improvements in mfERG recordings. When patients were evaluated individually 46% experienced an improvement in vision, 42% remained stable, and 12% worsened during the follow-up period. [3] Because this is the first study in pediatric patients the main objective is to report the safety results and there were no serious ocular and systemic complications in pediatric patients as reported in adults. This gives hope for studies including patients with the beginning/medium phase of their disease. In this current study, most of the treated eyes (75%) showed improvement in VA, perimetry, and electrophysiological results and the remaining treated eyes (25%) stayed stable whereas the majority of fellow eyes of the patients showed detoriation in visual functions. The improvements in functional and structural measurements were consistent during the first-year follow-up. It is suggested that stem cell-based therapies work better when there is more viable tissue and so theoretically pediatric patients having more residual visual capacity, the results are expected to be better than adults. When we compare the ratio of patients who gained visual acuity after MCS therapy, it was higher in pediatric patients (75%) than the adults (46%). [3] In our study group none of the patients experienced vison loss in the treated eyes during the follow-up period. However, the studies in adults reported detoriation in vision in 12% [3] and 18.7% [18] of the treated patients. We believe that this difference was due to the late stage of the disease in adults. These are the limitations to our study. We know that the study population is small and the fıllow-up period is short considering that this is a stem cell study. Furthermore, the study included children so visual field examinations or mfERG recordings may be unreliable because of poor compliance even they repeated the tests multiple times before the baseline evaluations. More objective tests like full field stimulus test and/or multiluminance mobility test should be included in studies including subjects with low vision.
5. Conclusions
- Stem cell treatment with suprachoroidal implantation of UCMSCs in children seems to be safe and effective. To optimize the interventional timing, site of graft replacement and delivery system future studies including larger number of cases with better visual acuities and with longer follow-up periods will be necessary.Although there are still many issues to be addressed before the final approval of MSC therapy for retinal degenerative diseases, the results obtained so far in preclinical animal models and in clinical trials are promising and encouraging. Therefore, the stem cell-based therapy offers a prospective option, especially for the patients without alternative therapeutic options. However, before the definitive expansion of the clinical use of MSC-based therapies, several questions, such as the sources of MSCs, the conditions of the in vitro propagation of MSCs, the routes of the cell applications or the possibility of the use of MSC products have to be answered and carefully verified.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML