-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Stem Cell Research
p-ISSN: 2325-0097 e-ISSN: 2325-0089
2022; 4(1): 1-7
doi:10.5923/j.ajscr.20220401.01
Received: May 11, 2022; Accepted: May 27, 2022; Published: Jun. 13, 2022

Bone Marrow Mesenchymal Stem Cells Differentiation after Feeding Rats with Magnesium Chloride: Potential Journey toward Osteogenic Microcosm?
Hossein Rafiei1, 2, Bashir Sobhani3
1KROK University, Kyiv, Ukraine
2PhD in Cell and Developmental Biology, Department of Biology, Faculty of Sciences, Islamic Azad University, Shiraz, Iran
3PhD Student in Comparative Histology, Department of Basic Sciences, Ferdowsi University of Mashhad, Iran
Correspondence to: Hossein Rafiei, KROK University, Kyiv, Ukraine.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Magnesium heavy metal is the fourth most abundant cation in the body, affecting different tissues directly or indirectly, such as bone marrow. In this paper, we exposed the rat's body to Magnesium Chloride at the dose of 12.5, 25, Control (50), 50, 100, 200, and 400 mg/l (ppm) in drinking water, then, bone marrow mesenchymal stem cells (BM-MSCs) were isolated and differentiated in a standard osteogenic medium. Initially, we divided rats into seven groups. The duration of feeding with Magnesium Chloride was two months; next, we harvested them. Then we isolated and cultured the bone marrow stem cells of the femur in all groups in the standard osteogenic medium for 21 days. Finally, we analyzed Osteocalcin gene expression and mineralization by real-time PCR and Alizarin red. Real-time PCR illustrated that Osteocalcin gene expression of rat BM-MSCs in 12.5 ppm group to 400 ppm group increased. Also, mineralization from 12.5 to 100 ppm had a rising tendency, whereas 200 and 400 ppm had an intensive reduction. It seemed that Magnesium metal accumulation with the doses mentioned In vivo led to the rise of Osteocalcin gene expression and decreased mineralization after differentiation of BM-MSCs in an osteogenic medium, Thus offering fresh outlooks for regenerative medicine applications to determine the appropriate conditions to get intended cells.
Keywords: Magnesium Chloride, Osteocalcin, Osteogenic medium, BM-MSCs, Real time PCR
Cite this paper: Hossein Rafiei, Bashir Sobhani, Bone Marrow Mesenchymal Stem Cells Differentiation after Feeding Rats with Magnesium Chloride: Potential Journey toward Osteogenic Microcosm?, American Journal of Stem Cell Research, Vol. 4 No. 1, 2022, pp. 1-7. doi: 10.5923/j.ajscr.20220401.01.
Article Outline
1. Introduction
- Magnesium is the fourth most plentiful cation in the body. It has various functions such as cofactor in the structures of many enzymes, nucleic acids, or mitochondria, and regulating a wide range of the activities like neuromuscular conduction, muscle contraction, blood pressure, myocardial contraction, glycemic control (Al Alawi et al, 2018), and bone formation (Orchard et al, 2014, Gröber et al, 2015). Magnesium depletion is associated with reduced osteoblastic and osteoclastic activity, osteopenia, bone fragility (Orchard et al, 2014). Universal drinking water and beverages contain moderate to much Magnesium (10–100 mg/l) (Rosanoff, 2013). Nuts, seeds, and unprocessed cereals contain high levels of Magnesium. Fruit, legumes, meat, and fish have intermediate Magnesium concentrations, whereas dairy products contain low levels (Jahnen-Dechent and Ketteler, 2012).Stem cells are unspecialized cells in two primary forms of embryonic and adult (Zakrzewski et al, 2019). The cells are recognized by their self-renewal capacity and much plasticity, and the cells can proliferate mature progenitor cells maintaining homeostasis processes actively via replacing damaged tissues cells (Rafiei et al, 2017). Several tissues contain mesenchymal stem cells (MSCs) such as liver, kidney, skin, adipose tissue, and bone marrow. One of the primary sources of stem cells is Bone marrow (Pittenger, 2008). Bone marrow MSCs (BM-MSCs) constitute a specific population of adult stem cells with multiple characteristics that can activate the mechanisms of repair after injuring tissue (Charbord, 2010). Some experimental models In vivo and a few clinical trials in humans powerfully illustrate that MSCs can regenerate bone and cartilage tissues (Chamberlain et al, 2007).Osteocalcin or bone γ-carboxyglutamic acid is an essential non-collagenous protein and bone endocrinology factor which is expressed and secreted exclusively by osteoblasts. After synthesizing protein, the mature peptide was firstly spliced several times. Then its three glutamic residues (located in positions 17, 21, and 24) in glutamic acid are carboxylated, forming a peptide with a high tendency to deposit in the bone and the extracellular matrix (Moser et al, 2019). Osteocalcin is the primary marker closely associated with bone formation during the treatment of osteoporosis disease. The knock-out of Osteocalcin leads to severe negative effects on the skeletal system and illustrates higher bone mineral density without any alteration in bone resorption and mineralization (Bi et al, 2017).The skeleton needs strength and structure via mineralization. The mineralization in bone also acts as a reservoir and plays a substantial role in calcium metabolism (Sato et al. 2019). Reduced availability of calcium or phosphate results in devastating mineralization in bone diseases such as rickets in children and osteomalacia in adults (Michigami, 2019).
2. Materials and Procedures
- Animals and Housing:In this experimental study, 70 male Rattus rattus (150 g) were chosen from Razi Vaccine and Serum Research Institute and randomly divided into 7 experiment groups. The animals were kept in polypropylene cages with a standard diet in a room containing well-ventilation with a 12:12 hour light/dark cycle. The experiment was based on the European Community guidelines (EEC Directive of 1986; 86/609/EEC) and the US guidelines (NIH publication # 85-23, revised in 1985). The Control group was fed by libitum feed and drinking water containing Magnesium Chloride with the dose of 50 ppm (permissible dose of WHO). Other groups tested were fed by libitum feed plus drinking water containing Magnesium Chloride with the doses of 12.5, 25, 50, 100, 200, and 400 ppm.BM-MSCs Isolation and Incubation:After two months of treatment, diethyl ether was used for anesthetizing the rats. BM-MSCs of the femur were isolated in a laminar flow hood with sterile conditions. Dulbecco's Modified Eagle Medium (DMEM) with 15% fetal bovine serum (FBS) (Sigma, USA) (Sigma, USA) and 1000 U/ml of penicillin G was applied for culture. Then the cells were incubated at 5% CO2 at 37°C (Smajilagić et al, 2013). Before differentiation, Real-time polymerase chain reaction analysis of pluripotency markers Nanog, Sox2, Rex1, and Oct4 was performed (Fafián-Labora et al, 2015).Osteogenic Culture:After obtaining much BM-MSCs, the cells were incubated in an osteogenic medium containing DMEM with 0.5 M ascorbate 2-phosphate (Sigma), 50 ng/ml thyroxine (Sigma), 1 nM dexamethasone (Sigma), 20 mM glycerol phosphate (Sigma, St Louis, MO), 100 μg/ml streptomycin, 12 mM glutamine, 10% fetal calf serum (FCS), 10% human serum (HS), 100 U/ml penicillin, 100 μg/ml streptomycin for 21 days. The cell medium was substituted every three days, then harvested and isolated RNA (Kulterer et al, 2007).RNA Isolation:The protocol outlined in the illustra™ RNAspin Mini RNA Isolation Kit (GE Healthcare, UK) with special manufacturers guidelines was used for total RNA isolation. RNase-free H2O with an amount of 40 μl was applied for RNA elution. For 10 min, the RNA was incubated at room temperature, then centrifuged at 11 000× g for 60 seconds. The RNA was Instantly put on ice to prevent degradation. The eluted RNA was maintained at - 20°C (Sahoo et al, 2017).cDNA Synthesis and Real Time PCR:The sequences of forward and reverse primers for the Osteocalcin gene and GAPDH gene are shown in table 1. GAPDH was a reference gene for normalizing in relative real-time PCR. cDNA was synthesized by Revert Aid H Minus first standard cDNA synthesis kit, Fermenras (Thermo Scientific™, Catalog number: K1631), based on manufacturer instructions. RNA incubation with an amount of 10
 was done at 65°C for 5-10 minutes. Then the following materials were mixed, showing table 2. The materials incubation was done at ten minutes at 25°C, then for 60 min at 42°C. For stopping the reaction, the mixture was left at 70°C for 10 min. The synthesized cDNA loading was at 80°C. In the end, QuantiTect SYBER Green PCR kit with a Bio-Rad detector system was applied for Real-time PCR.
was done at 65°C for 5-10 minutes. Then the following materials were mixed, showing table 2. The materials incubation was done at ten minutes at 25°C, then for 60 min at 42°C. For stopping the reaction, the mixture was left at 70°C for 10 min. The synthesized cDNA loading was at 80°C. In the end, QuantiTect SYBER Green PCR kit with a Bio-Rad detector system was applied for Real-time PCR.
|
|
3. Results
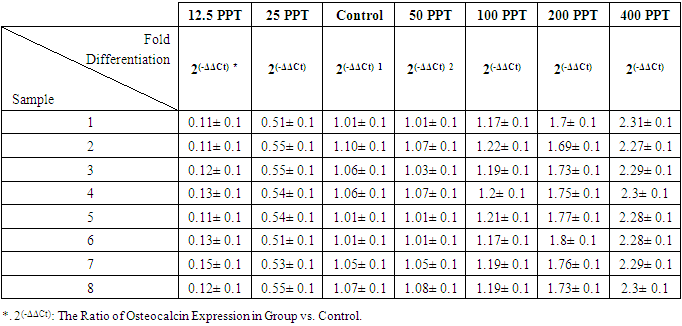
- Real time PCRThe real-time PCR results demonstrated that the Osteocalcin gene expression was most in the 400 ppm Magnesium Chloride group. It was approximately more than 20 times the group with 12.5 ppm and more than twice the group Control. The gene expression rose in the 25 ppm Magnesium Chloride group about five times more than in the first group. However, gene expression in the following groups, including 50, 100 and 200 ppm groups, showed an upward trend. The gene expression in the 100 ppm Magnesium Chloride group elevated approximately twelve times more than the 12.5 ppm group, whereas it slightly increased compared to the Control group. Also, The gene expression in the 200 ppm Magnesium Chloride group rose nearly fifteen times more than the group containing 12.5 ppm and one and a half times more than The Control group. There are significant Osteocalcin gene expression differences between groups (P <0.05) (Table 3 and Fig 1).
|
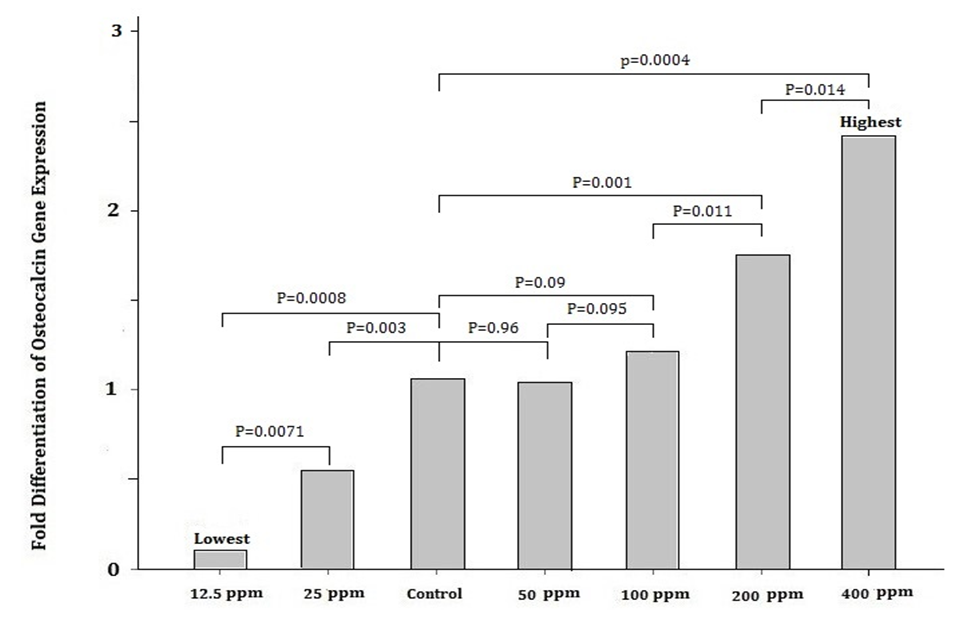 | Figure 1. Comparison between the Osteocalcin Gene Expression Median of MSCs in the Groups (P <0.05) |
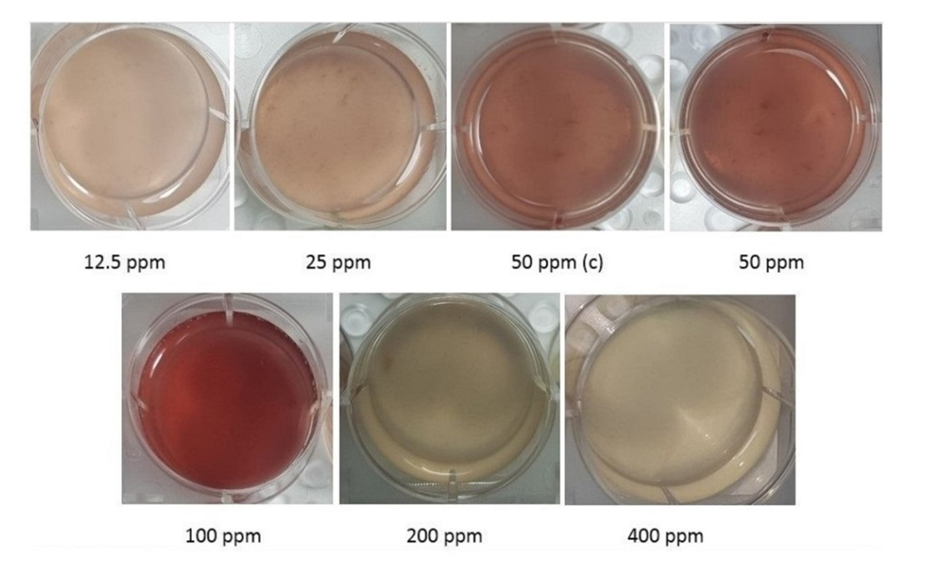 | Figure 2(a). Comparison of Alizarin red S Color of MSCs between the Groups (P <0.05) |
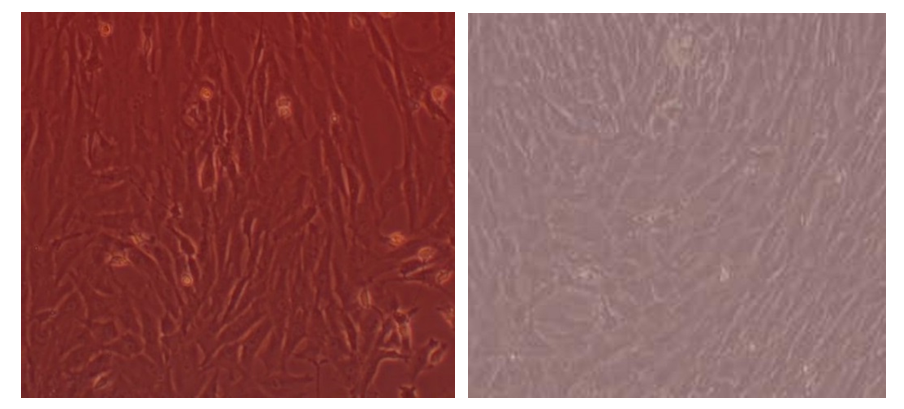 | Figure 2(b). Comparison of Color of MSCs Between the Most Alizarin red S Color (100 ppm) and the Least (400 ppm) |
4. Discussion
- Cell-based treatment for the repair of bone suggests a promising therapy for genetic musculoskeletal disorders and degenerative diseases. Although considerable advances were achieved with the help of stem cells and osteogenic medium, the former In vivo environment of the stem cells from which they were isolated is also substantial. In our study, we analyzed Osteocalcin gene expression and mineralization after the differentiation of BM-MSCs in the Osteogenic medium so that beforehand, the rats were respectively fed by doses of 12.5, 25, Control, 50, 100, 200, and 400 ppm of Magnesium Chloride for two months. Magnesium metal depletion In vivo is related to a reduction of osteoblast and osteoclast activity leading to osteopenia, bone fragility, and vitamin D decline in women during postmenopausal conditions (Serguienko et al, 2018). Although the evidence is still not comprehensive, most clinical research indicates that Magnesium is a contributor agent to bone health (Castiglioni et al, 2013). Magnesium ions/macrophage-conditioned medium lead to enhance osteogenesis of BMSCs via BMP/SMAD signaling. The manipulation of the Magnesium ion concentration of the stem cells medium creates the Magnesium biomaterial with desirable osteo-immunomodulatory characteristics leading to ameliorating and modifying the influences of Magnesium-based bone biomaterials (Zhang et al, 2019). Magnesium chloride promotes MSC proliferation by increasing Notch1 signaling pathway, and increasing the expression of Osteocalcin and induces osteogenic differentiation (Díaz-Tocados et al, 2017). Late marker genes in MSCs like Osteocalcin, Osteopontin, and bone morphogenetic protein 2 (BMP-2) in the presence of Magnesium elevate compared to standard differentiation medium (Tsao et al, 2017). Magnesium ions / macrophage-conditioned medium lead to enhance osteogenesis of BMSCs via BMP/SMAD signaling. The manipulation of the Magnesium ion concentration of the stem cells medium creates the Magnesium biomaterial with desirable osteo-immunomodulatory characteristics, leading to improving and modifying the influences of Magnesium-based bone biomaterials. (Zhang et al, 2019). Some factors play a considerable role in the osteogenesis of stem cells. We focused on both the Osteocalcin gene expression and mineralization in various Magnesium doses. There is conflicting data in the relation between Osteocalcin gene expression and mineralization. For instance, despite the necessary presence of Osteocalcin in initiating the formation process of hydroxyapatite crystals, there is an evidence showing that it is an inhibitor of bone mineralization via preventing precipitation of calcium salts in the high level Magnesium quantity (He et al, 2016; Zoch et al. 2016). Another finding depicts that the influence of Osteocalcin on hydroxyapatite formation by showing that osteocalcin regulates the mineral species maturation during osteogenic differentiation of mesenchymal stromal cells (Tsao et al, 2017). On the contrary, other experiment shows that Osteocalcin has no role in bone formation, resorption, and bone mass in estrogen-sufficient or estrogen-deficient conditions. Instead, it is vital for the alignment of biological apatite crystallites parallel to collagen fibers. (Manolagas, 2020). Convenient regulation of Mg2+ concentration over time is vital for normal biomineralization (Zhang et al, 2019). In normal osteoblasts, Osteocalcin expression rises when the supplemented Mg2+ is between 0.5 mM-2.0 mM while supplemented Mg2+ concentrations of 4mM lead to diminished expression (He et al, 2016). On the contrary, in our experiment, up to 400 ppm led to increasing Osteocalcin gene expression.Some data offer that Magnesium alone affects proliferation or delays the natural fate of Mesenchymal stem cells to mature and differentiate. However, during osteogenic differentiation, the influence of Magnesium becomes complicated and positive or negative which is related to several involved pathways (Luthringer et al, 2016). Exposure In vivo to high levels of Magnesium declines mineralization activities, for instance, the mineral to matrix balance, bone mineral crystallization, reducing bone quality and biomechanical function (Chu et al, 2020). Medium containing the high concentration of Magnesium regulates gene expression of MSCs during osteogenic differentiation leading to suppressing the mineralization process. Knockdown of Magnesium transporter SLC41A1 that controls the interaction of Magnesium and MSCs via Wnt signaling leads to elevating mineralization during osteogenic differentiation (Tsao et al, 2017). Unlike this experiment, Magnesium in the osteogenic medium in our experiment was standard, and we just regulated its levels In vivo.In our experiment, Osteocalcin gene expression and mineralization of stem cells from 12.5 to 100 ppm had an upward trend. The most level of mineralization was at 100 ppm, while there was an increase in Osteocalcin gene expression the doses of 200 and 400 ppm. The most level of the Osteocalcin expression was shown at the dose of 400 ppm. On the contrary, mineralization had a sudden decrease in 200 and 400 ppm, so that the least color staining of mineralization was in the dose of 400 ppm. Also, the mineralization of the 200 ppm group was less than the 12.5 ppm group.Magnesium has a roughly estimated biological half-life of ∼1000 h (42 days) (Schwalfenberg and Genuis, 2017). According to this, it is possible that Magnesium or at least the significant concentration of Magnesium does not leave the cells during the cell culture period and affects gene expression. Concerning paradoxical results about Magnesium in osteogenic differentiation, the investigation of various levels of Magnesium along with other factors affecting bone processes can be the best way to understand its precise roles.
5. Conclusions
- The heavy metal Magnesium intake affects bone marrow, promoting Osteocalcin gene expression and decreasing mineralization. Concerning Magnesium's half-life, it seems that at least its significant concentration did not leave the cells during the cell culture period and is effective in differentiation. Therefore, it seems necessary for In vivo regulation before isolating and differentiating the cells. These results propose novel perspectives in stem cell research showing that by regulating In vivo conditions, more optimal plasticity can be obtained in the stem cell differentiation In vitro.
Potential Conflict of Interest
- The authors have no conflicting financial interests.
ACKNOWLEDGEMENTS
- We would like to thank Bahman Rafiei and Robert Earl Nickerson. We declare that there is no conflict of interests regarding the publication of this paper.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML