-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Stem Cell Research
p-ISSN: 2325-0097 e-ISSN: 2325-0089
2019; 3(1): 1-7
doi:10.5923/j.ajscr.20190301.01

Treating Cervical Disc Pathology with Bone Marrow Concentrate is Equal or Better than Fusion or Disc Replacement at Two-Year Follow-up
Kenneth A. Pettine, Maxwell Dordevic
Celling Biosciences, USA
Correspondence to: Kenneth A. Pettine, Celling Biosciences, USA.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

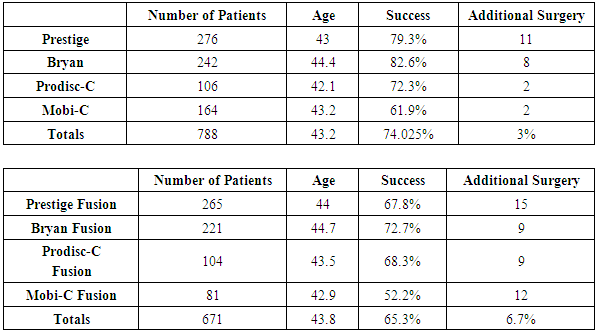
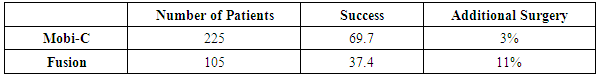
Objective: Millions of patients suffer chronic neck pain, headaches, inter-scapular pain, and radiating arm pain from degenerated cervical discs. Operative options include cervical disc fusion or cervical artificial disc replacement. Patients with more than two degenerated discs have minimal surgical options. Study Design: This is a prospective nonrandomized study of the two- year follow-up results of injecting bone marrow concentrate (BMC) into symptomatic degenerated cervical discs compared to five FDA studies comparing Cervical Artificial Disc Replacement (CADR) to Anterior Cervical Fusion (ACF). The BMC study is class two data. The FDA studies are class one data. Methods: There were 182 patients in the BMC study. The 30- minute procedure involved aspirating 55ml of bone marrow from the iliac wing, concentrating this via centrifugation to a volume of 3ml, and then injecting 0.5ml of the bone marrow concentrate into each abnormal cervical disc. The FDA studies involved 788 CADR and 671 ACF one level patients. There were 225 two-level CADR and 105 fusion patients. All the studies had a 2 -year follow-up. Inclusion/exclusion requirements were similar in all the studies. All of the studies similarly compared clinical outcomes. Results: The average NDI improved 63% and VAS 67% in the BMC study. All scores had a P-value of less than 0.001. There was no difference in the clinical results comparing one, two, three, four or five-disc levels injected. There were no injection complications, and no patient was made worse. The overall success in the CADR one level studies was 74% with a reoperation rate of 3%. The one level ACF had a success of 65.3%, and reoperation of 6.7%. The two-level CADR had a success of 69.7% and a reoperation rate of 3%. The ACF patients had a success of 37.4% and a reoperation rate of 11%. Conclusions: These results indicate a BMC injection may be a reasonable non-surgical option for patients with symptomatic degenerated cervical discs, especially in the multi-level abnormal disc patients.
Keywords: Mesenchymal Stem Cells, Stem cells, Cell-based therapy, Bone Marrow Concentrate
Cite this paper: Kenneth A. Pettine, Maxwell Dordevic, Treating Cervical Disc Pathology with Bone Marrow Concentrate is Equal or Better than Fusion or Disc Replacement at Two-Year Follow-up, American Journal of Stem Cell Research, Vol. 3 No. 1, 2019, pp. 1-7. doi: 10.5923/j.ajscr.20190301.01.
1. Introduction
- Millions of patients in the United States suffer from chronic complaints of neck pain, pain in between their scapulae, radiating pain producing headaches, and radiating pain into the arms. Epidemiologic studies have found the incidence in the general population to range from 7% to 13.8% [1, 2]. Cervicogenic headaches are considered the most common etiology for chronic headaches [3].Non-operative treatments for patients who have degenerative discs in their cervical spine with associated neck pain, headaches, pain in between their shoulder blades, and radiating arm pain can include traction, chiropractic care, physical therapy, acupuncture, epidural steroid injections, intermittent use of a soft collar, and ergonomic pillows for sleeping. Patients who continue to suffer severe symptoms may be candidates for a surgical approach to their problem.The standard surgical treatment for degenerative conditions of the cervical spine is an anterior cervical fusion [4-8]. One inherent problem with any fusion of the spine is the permanent loss of spine motion and the development of adjacent level abnormalities [9-12]. Research has indicated the development of adjacent level abnormalities leading to additional surgery is between 3% and 5% per year after an anterior cervical fusion [13]. Several review articles of the literature indicate the clinical success rates of anterior cervical fusion at one level are about 70% with a reoperation rate at the two-year follow-up of 10% [14]. The clinical results of anterior cervical discectomy and fusion (ACDF) decrease the more levels that are fused [15, 16]. Another inherent problem with anterior cervical fusion is the failure of the fusion to heal. This results in what is called a pseud-arthrosis or failure of fusion. Half the patients with this situation generally require a second surgery in an attempt to obtain a fusion [17, 18].Cervical artificial disc replacement (CADR) is a newer surgical option. This procedure underwent FDA testing beginning around 2005. McAfee et al., published a meta-analysis comparing outcomes of artificial cervical disc versus anterior cervical fusion at one level. This meta-analysis reported superior reports with the use of an artificial disc versus a fusion at a single level when considering adjacent level degeneration [19]. Recently, an artificial disc has been approved for two levels. The prospective randomized study supporting this also indicated superior results with an artificial disc versus fusion at two levels [20]. There have been numerous papers published indicating the clinical superiority of artificial cervical disc, over fusion subsequent to 2005 [21-27]. One persistent problem, however, is that many patients have degenerative changes at more than two levels, which is a prognostic indicator of poorer outcomes in surgical procedures.Patients who have three or more degenerated discs in the cervical spine present a challenging surgical treatment situation. The surgical results of three- and four-level anterior cervical fusions are certainly less than the 65% success rate reported with fusion at a single level. Thus, patients with more than two levels of degenerative changes in their neck have very poor surgical options [15, 16]. Performing CADR at more than two levels is unusual and would very rarely be covered under insurance benefits.The use of biologics to treat disc abnormalities is a possible non-surgical option which potentially can bridge the gap between traditional non-surgical treatments for cervical degenerative disc abnormalities and surgery. There is mounting evidence to support the use of biologic and cell-based therapy for chronic discogenic low back pain, a condition with similar etiology [28, 29]. The authors of this paper have published one, two and three- year follow-up from a study assessing the safety and efficacy of bone marrow concentrated cells as an alternative to surgery for discogenic back pain at one or two levels [30, 31]. There have been numerous studies utilizing mesenchymal stem cells to enhance tissue repair and decrease inflammatory damage in both in vitro lab studies and in vivo clinical models [32-36]. It is known that bone marrow concentrate (BMC), the treatment used in this study, contains mesenchymal stem cells as well as a number of other cell types including but not limited to: hematopoietic stem cells, endothelial progenitor cells, and platelets. Studies have shown both the mesenchymal stem cell population and other nucleated cell types have healing properties and may contribute synergistically to the healing seen in studies on the spine [37-44].This is the first study to evaluate the clinical results of intradiscal BMC to treat patients who have symptomatic degenerated cervical discs and associated chronic axial neck pain, headaches, and radiating arm pain, compared to anterior cervical fusion and artificial cervical disc replacement.
2. Materials and Methods
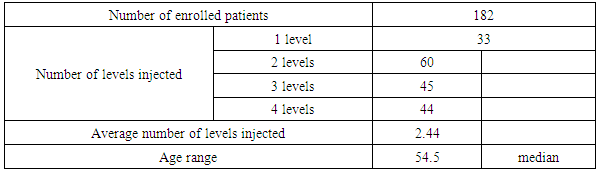
- Study DesignThis study is a prospective open-label non-randomized evaluation of patients having an injection of BMC (class two data) compared to ACDF and CADR. The ACDF and CADR studies were prospective randomized studies (class one data). Follow up in all the studies was two years. The patients enrolled as subjects in all these studies presented clinically with symptomatic moderate to severe chronic axial neck pain. Axial neck pain was also associated with inter-scapular pain, headaches, and radiating arm pain. Abnormalities were present on cervical MRI scanning and plain radiographs. These abnormalities include anterior and posterior osteophyte formation, disc space narrowing on plain radiographs, and nucleus pulposus desiccation on MRI scanning.Pre-treatment baseline neck disability index (NDI) was a minimum of 30mm/100mm and pre-treatment baseline axial neck pain was at least 40mm/100mm on visual analog scale (VAS) pain scores. The patients were required to sign and fully comprehend an informed consent document before participating in the study. All patients underwent a pre-injection medical history and physical examination along with the neck disability index and visual analog scale pain scores.These questionnaires were repeated at six weeks, three months, six months, 12 months, and 24 months post injection of BMC. The patients’ primary physical complaint in this study was one of axial neck pain with associated inter-scapular and headaches and may or may not have included radicular arm pain. Standard exclusion criteria included evidence of a symptomatic herniated disc. Patient demographics and treatment levels for the BMC study are listed in Table 1. The Inclusion/exclusion requirements were similar in all FDA IDE studies, and this study except the FDA studies required a neurologic deficit and accepted patients with a herniated disc.
|
3. Results
- BMC Injection Results: Pre-procedure neck disability index (NDI) median was 44.5, and visual analog scale (VAS) median was 62. Two-year follow-up NDI and VAS were 16.5 and 20.7. All scores had a p-value of less than 0.001. This represents a 63% improvement in NDI and a 67% improvement in VAS at the two -year follow up. There was no difference in the clinical results comparing one, two, three, four or five-disc levels injected. There were no injection complications, and no patient was made worse by the procedure. No patient had surgery during the study. Figure 1 details the pre-procedure and post-procedure changes in NDI and VAS through two-year follow-up. The results in Figure 1 represent all 182 patients.
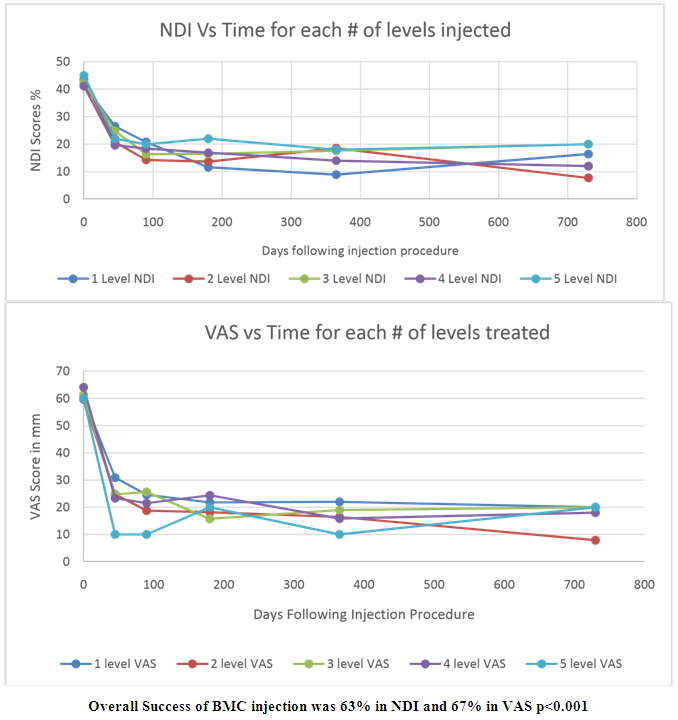 | Figure 1. Clinical results of the BMC Injection |
|
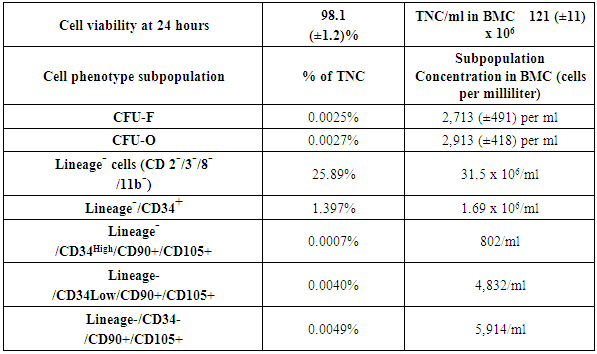
 Analysis of the Bone Marrow ConcentrateThis section is included from a previously published paper to detail the BMC cell analysis expected in these patients [30]. The demographics of those patients were similar to this study of patients with the same diagnosis in the cervical spine. This information is included to detail the method of cell analysis and MSC cell counts expected in this group of 182 patients.An aliquot (1ml) of each subject’s BMC was packed in a shipping container with 5°C cold packs and shipped overnight to the cell analysis laboratory (Celling Biosciences, Austin, TX). The samples were received and processed immediately to determine total nucleated cell (TNC) count and viability using a Nucleo-Counter NC-100 (Chemo-metric, Denmark). The BMC was diluted in phosphate buffered saline (PBS, Invitrogen, Grand Island, NY) with 2% fetal bovine serum (FBS, HyClone human mesenchymal grade, Thermo Scientific, Waltham, MA) and subjected to a Ficoll-Paque (GE Healthcare Life Sciences, Piscataway, NJ) gradient separation (1:1 cell solution to Ficoll ratio by volume) in order to deplete red blood cells. Analysis of the recovered cells included performing colony-forming unit-fibroblast and osteogenic (CFU-F and CFU-O, respectively) assays and phenotypic analysis by flow cytometry. For phenotype analysis, fresh (non-cultured) BMC cells were stained with a series of rabbit anti-human monoclonal antibodies for a hematopoietic lineage-committed (non-progenitor) panel of markers including CD2, 3, 8, and 11b (APC-Cy7), CD34 (PE), CD90 (FITC), and CD105 (APC) as well as appropriate isotype controls. Isotype, single color stain, and four-color stain samples were analyzed by a Guava Easy-Cyte 8HT (Millipore, Billerica, MA). The CFU-F assay was performed by creating a dilution series (in culture medium with 5% FBS and 1% antibiotics) of each cell preparation at concentrations of 50,000-500,000 TNC per well in standard 12-well plates. The plates were placed in an incubator at 37°C, 5% CO2, and 100% humidity for 72 hours when the medium was replaced. The medium was replaced every three days. After nine days in culture, cells were gently washed with PBS, fixing the colonies/cells with methanol, staining the attached cells with Crystal Violet, rinsing with water, and air-drying the plates. Visualization and counting of the colonies were done with an inverted microscope. Colonies containing 20 or more cells were scored as a CFU-F. The CFU-O assay was performed identically as CFU-F, but after nine days the medium was changed to an osteogenic induction medium (Advance STEM Osteogenic Differentiation Kit, Hy-Clone, Logna, UT) for an additional nine days with complete medium change every three days. On day 18, the wells were washed with PBS, then fixed for 15 minutes in 2% formalin solution, and contained for alkaline phosphatase activity (Vector Blue ALP, Vector Labs, Burlingame, CA) and calcified extracellular matrix (0.5% Alizarin Red solution, Sigma-Aldrich, St. Louis, MO).Outcome Assessment and AnalysisThere were no serious complications from harvesting the bone marrow concentrate or the disc injections. The most common events were transient pain at the harvest site and discomfort at the injection site, both of which typically resolved within 48 hours of treatment. Not every patient improved significantly, but no patient reported increases in visual analog scale or neck disability index from pretreatment scores. Patient follow-up outcomes were obtained by independent reviewers who were not investigators with the study. The reviewers were paid, senior pre-med students. Uni-variable data comparisons of baseline to follow-up were analyzed using a two-tailed student’s t-test with a 95% confidence interval (Alpha=0.05, Microsoft Excel).Demographic comparisons were made using paired sample tests
Analysis of the Bone Marrow ConcentrateThis section is included from a previously published paper to detail the BMC cell analysis expected in these patients [30]. The demographics of those patients were similar to this study of patients with the same diagnosis in the cervical spine. This information is included to detail the method of cell analysis and MSC cell counts expected in this group of 182 patients.An aliquot (1ml) of each subject’s BMC was packed in a shipping container with 5°C cold packs and shipped overnight to the cell analysis laboratory (Celling Biosciences, Austin, TX). The samples were received and processed immediately to determine total nucleated cell (TNC) count and viability using a Nucleo-Counter NC-100 (Chemo-metric, Denmark). The BMC was diluted in phosphate buffered saline (PBS, Invitrogen, Grand Island, NY) with 2% fetal bovine serum (FBS, HyClone human mesenchymal grade, Thermo Scientific, Waltham, MA) and subjected to a Ficoll-Paque (GE Healthcare Life Sciences, Piscataway, NJ) gradient separation (1:1 cell solution to Ficoll ratio by volume) in order to deplete red blood cells. Analysis of the recovered cells included performing colony-forming unit-fibroblast and osteogenic (CFU-F and CFU-O, respectively) assays and phenotypic analysis by flow cytometry. For phenotype analysis, fresh (non-cultured) BMC cells were stained with a series of rabbit anti-human monoclonal antibodies for a hematopoietic lineage-committed (non-progenitor) panel of markers including CD2, 3, 8, and 11b (APC-Cy7), CD34 (PE), CD90 (FITC), and CD105 (APC) as well as appropriate isotype controls. Isotype, single color stain, and four-color stain samples were analyzed by a Guava Easy-Cyte 8HT (Millipore, Billerica, MA). The CFU-F assay was performed by creating a dilution series (in culture medium with 5% FBS and 1% antibiotics) of each cell preparation at concentrations of 50,000-500,000 TNC per well in standard 12-well plates. The plates were placed in an incubator at 37°C, 5% CO2, and 100% humidity for 72 hours when the medium was replaced. The medium was replaced every three days. After nine days in culture, cells were gently washed with PBS, fixing the colonies/cells with methanol, staining the attached cells with Crystal Violet, rinsing with water, and air-drying the plates. Visualization and counting of the colonies were done with an inverted microscope. Colonies containing 20 or more cells were scored as a CFU-F. The CFU-O assay was performed identically as CFU-F, but after nine days the medium was changed to an osteogenic induction medium (Advance STEM Osteogenic Differentiation Kit, Hy-Clone, Logna, UT) for an additional nine days with complete medium change every three days. On day 18, the wells were washed with PBS, then fixed for 15 minutes in 2% formalin solution, and contained for alkaline phosphatase activity (Vector Blue ALP, Vector Labs, Burlingame, CA) and calcified extracellular matrix (0.5% Alizarin Red solution, Sigma-Aldrich, St. Louis, MO).Outcome Assessment and AnalysisThere were no serious complications from harvesting the bone marrow concentrate or the disc injections. The most common events were transient pain at the harvest site and discomfort at the injection site, both of which typically resolved within 48 hours of treatment. Not every patient improved significantly, but no patient reported increases in visual analog scale or neck disability index from pretreatment scores. Patient follow-up outcomes were obtained by independent reviewers who were not investigators with the study. The reviewers were paid, senior pre-med students. Uni-variable data comparisons of baseline to follow-up were analyzed using a two-tailed student’s t-test with a 95% confidence interval (Alpha=0.05, Microsoft Excel).Demographic comparisons were made using paired sample tests
|
4. Discussion
- Several human studies have recently been published documenting the clinical results of utilizing biologics to treat symptomatic chronic discogenic pain. The Coric, Pettine study was an FDA phase one evaluation of utilizing expanded juvenile cartilage cells to treat disco-genic low back pain [47]. Fifteen patients were injected at one lumbar level with 10 million cells and followed for one year. ODI went from 53.3 to 20.3 (p-value<0.0001) and SF-36 improved from 35.3 to 46.9 (p-value<0.0002). MRI improvement of at least one Pfirrmann grade was observed in 77% of patients. No patient had surgery. Pettine et al., have published one, two, and three-year follow- up studies on 26 patients injected with BMC for disco-genic low back pain. The average improvement in ODI was 64%, and VAS was 71%. Only five of the 26 patients had surgery [30, 31].This study is a prospective non-randomized open-label evaluation of 182 patients followed for two years to obtain preliminary data on the safety and efficacy of utilizing BMC to treat symptomatic cervical degenerated discs compared to surgery. The BMC study included injections into one to five discs. There were four FDA studies of one level disc surgery and one study of two-level surgery.The results in this group of 182 patients undergoing a single injection of BMC into 1 to 5 discs in the cervical spine was unexpected. The two-year follow-up showed an average improvement in NDI of 63% and VAS of 67% (p<0.001). No patient was made worse, and no patient underwent surgery during the follow-up.Limitations of the BMC study include no randomized control, no follow-up MRI scan data, and no cell count data. The author has published MRI follow-up data and cell count data in a similar group of patients in the lumbar spine [30, 31]. Limitations of the FDA studies include a low percentage of follow-up from the original patients to the two -year follow-up.Patients with more than two levels of symptomatic disco-genic cervical pain have limited treatment options. There is minimal literature reporting the long-term efficacy of any non- operative treatment. Two-year follow-up data in treating multilevel discogenic cervical pain with the BMC showed an improvement in NDI of 63% (p<0.001) and VAS of 67% (p<0.001). No patient was made worse by the procedure, and there were no complications from the percutaneous injection of BMC into the disc. Utilizing MSCs derived from BMC, based on these preliminary results, may offer patients with multilevel disco-genic cervical pain a viable treatment option to surgery.
ACKNOWLEDGMENTS
- I would like to acknowledge the help of Dylan Merideth and Nick Collins in obtaining patient follow up.Funding- There was no outside funding for this researchConflict of interest- No is no conflict of interest with the authors and this research
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML