-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Stem Cell Research
p-ISSN: 2325-0097 e-ISSN: 2325-0089
2018; 2(1): 5-10
doi:10.5923/j.ajscr.20180201.02

Tibial Metaphysical Injection with Bone Marrow Concentrate to Treat Knee Arthritis
Kenneth Pettine M.D., Maxwell Dordevic
Research and Development, Celling Biosciences, Austin, USA
Correspondence to: Kenneth Pettine M.D., Research and Development, Celling Biosciences, Austin, USA.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Study Design: This is a prospective non-randomized study of the one-year follow-up results of injecting bone marrow concentrate (BMC) into the knee joint and medial tibial metaphysis of patients with a diagnosis of knee osteoarthritis (OA). This is a Class-2 consecutive cohort study. Methods: There were 23 patients in the BMC study. The 30-minute procedure involved aspirating 55ml of bone marrow from the iliac wing, concentrating this via centrifugation to a volume of 12ml and then injecting 6ml into the medial tibial metaphysis and 6ml into the medial knee joint compartment. All the patients had a pre-treatment standing radiograph and MRI. Clinical outcome was measured with a VAS and lower extremity functional scale (LEFS) at 6 weeks, 3, 6, and 12 months. Results: The average VAS improved 59% (p<0.001) and LEFS 47% (p<0.001). There were no injection complications and no patients were made worse. No patient had knee surgery during the study. Radiographs did not improve in any patient. MRI scans showed resolution of the medial tibial metaphyseal bone inflammation in 10/12 patients. Conclusions: These results indicate a BMC knee injection has safety and efficacy and may be a reasonable non-surgical option for patients with moderate to severe knee OA.
Keywords: Mesenchymal Stem Cells, Stem cells, Cell-based therapy, Bone Marrow Concentrate
Cite this paper: Kenneth Pettine M.D., Maxwell Dordevic, Tibial Metaphysical Injection with Bone Marrow Concentrate to Treat Knee Arthritis, American Journal of Stem Cell Research, Vol. 2 No. 1, 2018, pp. 5-10. doi: 10.5923/j.ajscr.20180201.02.
Article Outline
1. Introduction
- Osteoarthritis (OA) affects 50 million Americans [1]. The American Academy of Orthopedic Surgeons (AAOS) recommended treatments for osteoarthritis of the knee include the following: weight loss, gentle exercise, and anti-inflammatory medications followed by total knee replacement [19, 20]. The AAOS does not recommend arthroscopic debridement or any Hyaluronic acid products such as Synvisc®, Euflexxa™, Orthovisc®, Supartz™, or Hyalgan® for treating knee OA. [23] Four prospective randomized studies have shown no benefit over placebo at six-month follow-up with these Hyaluronic injections [35-38]. Despite the fact Hyaluronic acid products have shown no efficacy, the market for these products is over a billion dollars per year. [42] This is due to the huge void between non-operative treatments and the only surgical treatment, total knee arthroplasty [TKA].Last year, over 700,000 knee replacements were performed in the United States with a direct cost of over $20 billion [19]. These numbers are expected to double in the next three years. Every day, 10,000 people in the US turn 65 and this will continue for the next 14 years [21]. Currently, the US government is collecting objective data on the results of joint replacement surgery. This data comes from two studies: Osteoarthritis initiative (OAI) and the multi center osteoarthritis study (MAST). The conclusion of the OAI was, taken as a group and based on the cost, knee replacement surgery had minimal improvement in quality of life. [33] The MAST reviewed 1308 articles on the results of total hip and knee replacement surgery. One hundred and fifteen articles reported on pain outcomes. The proportion of people with an unfavorable long-term pain outcome in all these studies ranged from 10% to 34% after knee replacement surgery. In the best quality studies, an unfavorable pain outcome was recorded in 20% of patients after knee replacement. This study concluded that a significant proportion of people have pain after TKA surgery. The paper concluded, “There is an urgent need to improve general awareness of these statistics in the general public”. [34]Mesenchymal stem cells [MSC] obtained from bone marrow concentrate [BMC] have many positive attributes. MSCs are anti-inflammatory, secrete numerous growth factors, stimulate blood vessel formation, and modulate the body’s immune system to enhance healing [29, 43]. Delivering MSCs directly into the affected joint has been shown to provide therapeutic benefit. The use of BMC to obtain MSC is standard of care in the veterinary world for the treatment of arthritic joints primarily in dogs and horses. Several prospective, randomized, double blind studies have been published indicating the efficacy of utilizing autogenous mesenchymal stem cells for the treatment of OA in dogs [3, 4]. There are numerous other animal studies also verifying the efficacy of using BMC to treat OA. [7, 9-18, 22]Why does osteoarthritis cause knee pain? Recently, orthopedic surgeons hypothesize the pain of knee osteoarthritis may be caused by abnormal stresses in the metaphyseal bone beneath the tibial plateau, not cartilage degradation. MRI scanning in patients who have medial osteoarthritis of the knee can show an isolated hyperemic area just beneath the tibial plateau in the tibial metaphysis. This hyperemic area on T2 weighted imaging is consistent with bone inflammation resulting in a localized area of increased blood flow [2, 6, 13, 14, 17, 26-28, 30-32].Surgeons have injected the inflamed tibial metaphysis with various materials including bone cement and numerous types of bone grafting to decrease pain. It is known that MSCs can differentiate into osteoblasts. Based on this rationale, I began injecting patient's inflamed medial tibial plateau with BMC to potentially decrease knee pain. This treatment avoids the morbidity of injecting cement and doesn’t require bone grafting.
1.1. Objective of the Study
- The primary objective of the study was to determine the safety and efficacy of performing an intra-articular injection and injection into the medial tibial metaphysis of autogenous BMC cells into the symptomatic knee to treat medial compartment osteoarthritis.
1.2. Secondary Objectives
- 1. To evaluate the effectiveness of autogenous BMC in improving function and limiting disability as measured by the Lower Extremity Functional Scale (LEFS).2. To evaluate improvements in pain as assessed using a visual analog score (VAS) for pain.3. To evaluate the effectiveness of autologous BMC in reducing the need for surgical intervention out to one-year post-injection.4. To provide preliminary data to support the hypothesis that the pain of knee osteoarthritis may be bone inflammation and not entirely from cartilage degradation.
2. Materials and Methods
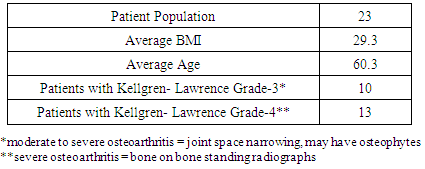
- This study is a non-IRB prospective open-label non-randomized consecutive case series evaluation of utilizing BMC for the treatment of medial OA of the knee. This study was performed at a single center. The patients all paid for the procedure. No patient dropped out of the study. Inclusion criteria required all of the patients to have complaints of knee pain consistent with only medial OA of the knee. The patients all had bilateral OA. Only the most symptomatic knee was treated. The non-treated knee served as a control. All patients had an isolated area of hyperemia on T2 weighted MRI in the medial tibial metaphysis. OA was defined by pain and stiffness in the medial side of the knee worsened with exercise and weight bearing. All patients underwent a pre-injection medical history and physical examination of their knee including a lower extremity functional scale score and visual analog scale pain score. In addition, all patients had standing AP and lateral radiographs as well as MRI scanning. Standing radiographs were utilized to rate the patients as 0, 1, 2, 3, or 4 on the Kellgren-Lawrence scale. [24] Follow-up was obtained at 3 months, 6 months, and 12 months following the procedure in all 23 patients.One Year follow-up standing radiographs were obtained and graded in every patient. Follow-up MRIs were obtained in 12 patients. Follow-up data was obtained by two independent research assistants with the results blind to the treating physician.
|
3. Bone Marrow Collection and Processing
- Bone marrow aspirate (BMA, 55mL) was collected over acid citrate dextrose-anticoagulant (ACD-A, 5mL) from the patient's posterior iliac crest. The procedure was performed with IV sedation consisting of Versed and Fentanyl. Positioning of the Jamshidi needle in the iliac wing was confirmed by fluoroscopy. BMA was collected in a 60mL syringe in a series of discrete pulls on the plunger (targeting a collection of 5-10mL per pull), with repositioning of the needle tip between pulls based on the reported enrichment of progenitor cells by Hernigou et al.[37]. The BMA was processed using the ART21 system (Celling Biosciences, Austin, TX) to produce a bone marrow concentrated cell preparation. A BMC volume of 12mL was drawn from the centrifuge. Six mL was used for the injection into the medial tibial metaphysis and 6mL was injected into the intra-articular medial knee joint.
3.1. Knee Injection
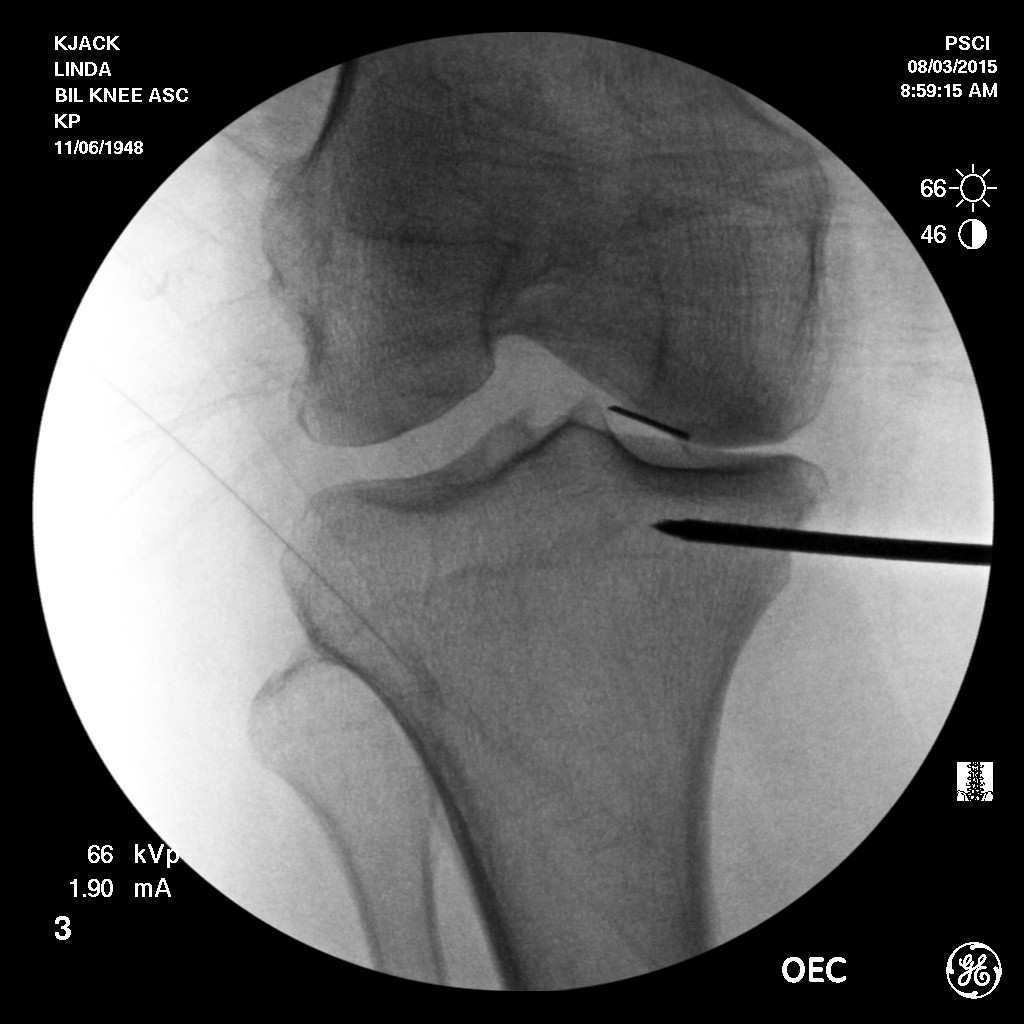
- With the patient in a supine position, the most symptomatic knee was sterilized with a Betadine skin prep. Under fluoroscopic control, a 20-gauge needle was placed medial to the peripatellar tendon into the medial tibial compartment. Needle placement was verified with fluoroscopy. At this point, 6mL of BMC was placed into the medial compartment of the knee joint. Following this, the medial skin, located 1.5cm below the tibial plateau, was anesthetized with buffered 1% Xylocaine. A Jamshidi needle was then advanced through the cortical bone of the tibial metaphysis 2cm into the medial tibial metaphysis approximately 1.5cm below the plateau. The needle was placed midline anterior posterior of the tibia with needle placement verified under fluoroscopic control. At this point, 6mL of bone marrow concentrate was slowly (1mm of BMC every 10 seconds) placed into the cancellous bone of the medial tibial metaphysis. This assured even distribution of the bone marrow concentrate into the tibial metaphysis and kept the pain of the injection to a minimum. A plug of bone wax was placed through the Jamshidi needle to plug the entry spot into the bone and prevent back leakage of the biologic material. The entire procedure averaged less than 45 minutes. Patients were prescribed pain medication as needed for three days and put on restricted physical activity for two weeks, passive, low resistant range of motion was encouraged immediately. At two weeks, the patients were allowed to return to full activities.
4. Results
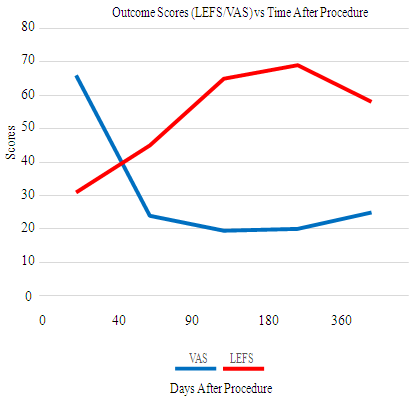
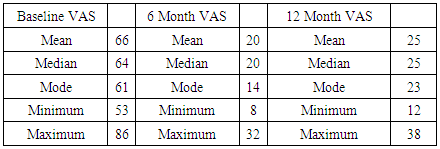
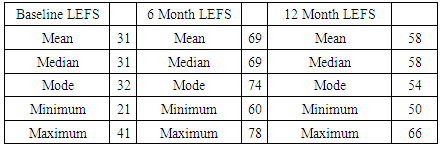
- No patient had knee surgery during the one-year follow-up. There were no serious adverse events reported, and no patient reported increases in pain or disability (VAS and LEFS score). Between 3 and 6 months 8 /23 patients reported VAS pain at or below 20. Only 4 had VAS pain scores above 30. There were significant improvements in Lower Extremity Functional Scale (LEFS) as well, with the average LEFS score (out of 80, with 80 being no limitation/disability and 0 being complete disability) improving from a 31 at baseline to an average 58 at one -year follow up (47% improvement in LEFS). The average VAS score dropped from 61 at baseline to 25 at one-year follow-up (59% improvement in VAS). There was no improvement in the standing radiographic appearance of the knee joint between pre-procedure and one-year follow-up in any patient (no change in Kellgren-Lawrence grade in any patient).Follow-up MRI scans showed a resolution of the metaphyseal hyperemia in 10/12 patients. No patient reported clinical improvement in the non-treated knee.
|
|
5. Discussion
- Progress in molecular biology has profoundly modified the theory that OA is totally the result of the break -down of articular cartilage. New theories have been introduced in favor of an "inflammatory" paradigm. Recent reports have shown that the subchondral bone may have a substantial role in the OA process. Initially considered cartilage driven, OA is now considered to be a much more complex disease with inflammatory mediators released by cartilage, bone and synovium. [37, 38] MSCs obtained from BMC have many positive attributes. MSCs are anti- inflammatory, secrete numerous growth factors, stimulate blood vessel formation, and modulate the body’s immune system to enhance healing [5, 38]. Delivering MSCs directly into the affected joint and into the inflamed subchondral bone may provide therapeutic benefit. BMC contains many enriched growth factors like VEGF, PDG-F, TGF-Beta, FGF, Alpha-2 Macroglobulin, Interleukin-1 receptor antagonist protein, and Fibrinogen. [39, 40, 43]This study also introduces the novel treatment approach of injecting the tibial metaphysis below the degenerated medial tibial plateau. MRI scanning of patients with medial OA of the knee often shows a hyperemic area near the medial tibial metaphysis on T2 weighted images. Patients will point directly to this area to describe their knee pain location and the medial tibial metaphysis is painful to palpation on physical examination. The hypothesis is that abnormal compressive forces during ambulation are transferred through the medial tibial femoral joint to the medial tibial plateau and metaphysis. These abnormal stresses can produce micro- fracturing. [2, 6, 13, 17, 26-28, 30-32]. The pathology is not avascular necrosis but the opposite of hyperemia.The rationale for injecting the tibial metaphysis is so the MSCs in the BMC will decrease the bone inflammation and differentiate into osteoblasts. These two actions of the MSCs may restore the damaged and inflamed medial tibial metaphysis to a normal physiologic structure. The goal of injecting both the knee joint and the medial knee joint and tibial metaphysis is to maximize the efficacy of BMC to treat knee OA.Limitations of this study include: small number of patients, one-year follow-up, lack of a randomized control, lack of cell count data, non-IRB supervision and follow up MRI scans in only 12 of the 23 patients.The study did have strict inclusion/exclusion criteria. This was a consecutive series and no patient dropped out of the study. The patients all paid for their treatment which if anything biased the results to emphasize the negative. The control in this study was the opposite knee since all the patients had evidence of bilateral OA. No patient reported improvement in the non-treated knee. Follow up MRI scans were only obtained in 12 patients because of a lack of study funding and inability to obtain insurance authorization. The authors have previously published cell count data from a similar group of patients [44].
6. Conclusions
- This study shows that injecting the knee joint and tibial metaphysis has safety and efficacy. The improvement in VAS and LEFS was statistically significant (p<0.001). The average patient improved 59% in VAS and 47% in LEFS. MRI scans showed resolution of the tibial metaphyseal hyperemia in 10/12 patients. No patient had knee surgery during the study. No patient was made worse from the biologic procedure.These results indicate much of the pain of knee OA may be from inflamed metaphyseal bone and not cartilage degradation. It may be appropriate for a patient with OA to have a knee joint and tibial metaphyseal injection of autogenous BMC prior to consideration of total knee arthroplasty.
ACKNOWLEDGEMENTS
- We would like to acknowledge the help of Dylan Meredith and Nick Collins in obtaining patient follow up.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML