-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Polymer Science
p-ISSN: 2163-1344 e-ISSN: 2163-1352
2025; 14(3): 39-45
doi:10.5923/j.ajps.20251403.01
Received: Sep. 27, 2025; Accepted: Oct. 25, 2025; Published: Oct. 31, 2025

Comparative Study on Cryogenic and Conventional Chitosan Production from Apis Mellifera Dead Honey Bees: Process Optimization, Characterization and Environmental Impacts
Kuchkorova D. U.1, Ikhtiyarova G. A.2, Berdikulov B. Sh.1
1PhD Student, Department of General Chemistry, Tashkent State Technical University named after Islam Kariomov, Tashkent, Uzbekistan
2Professor, Department of General Chemistry, Tashkent State Technical University named after Islam Kariomov, Tashkent, Uzbekistan
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The study compares the production of chitosan from Apis mellifera (dead honey bees) using cryogenic and conventional methods, finding that the cryogenic method saves time, energy and water while producing chitosan with similar physicochemical properties. In this research work, it was found that obtaining chitosan cryogenic method cost and time are saved to conventional method. The study also uses various analytical techniques such as SEM, TGA and DTG analysis to characteriza the produced chitosan. The obtaining chitosans were applied in the dyeing process.
Keywords: Apis mellifera dead bees, Chitin, Chitosan, Biomaterials, Pre-deacetylation steps, Chemical extraction, SEM and TGA analysis
Cite this paper: Kuchkorova D. U., Ikhtiyarova G. A., Berdikulov B. Sh., Comparative Study on Cryogenic and Conventional Chitosan Production from Apis Mellifera Dead Honey Bees: Process Optimization, Characterization and Environmental Impacts, American Journal of Polymer Science, Vol. 14 No. 3, 2025, pp. 39-45. doi: 10.5923/j.ajps.20251403.01.
Article Outline
1. Introduction
- Chitosan is a useful biopolymer obtained by alkaline deacetylation of chitin. Generally, chitin is converted into chitosan with various degrees of deacetylation (DD) and molecular weights (Mw) depending upon the purpose of chitosan utilization. Chitosan differs from chitin in that it is soluble in mild acidic medium. The cationic form of chitosan in acidic solution plays a role in not only governing its solubility but also acting as an active site. The applications of chitosan for improving dyeability of cotton/polyester blended fabric have been widely studied. In the textile area, the higher the active site of chitosan favors the higher the dye adsorption (including natural dye) as well as film formation on fiber surface. Chitosan film on fabric surface is not desirable since it causes the problem of fabric stiffness (poor handling). Fortunately, these effects could be adjusted by the usage of chitosan’s proper molecular weight. Normally, chitosan with various molecular weights could be achieved by depolymerization techniques [1,2]. Chitin is a polysaccharide made from N-acetyl-D-glucosamine units which is found mainly in crustacean shells, insects, and microorganisms such as fungi, algae, and yeasts [3]. Extracted chitin is classified as α, β, and γ-chitin according to anti-parallel, parallel, and alternated alignments of the polymer chains (as a combination of α and β structures), respectively. α-chitin is usually obtained from the crustacean exoskeletons, specifically from shrimps and crabs; β-chitin can be isolated from squid pens; and -chitin is obtained from fungi and yeast [4]. Crustacean shells and fungal mycelia are the industrial resources of chitin [3]; [5]. In Uzbekistan, the chitin shells of dead honeybees has also been recognized as a valuable source for the extraction of chitin and its derivative, chitosan, which are extensively utilized in diverse scientific and industrial fields [6,7,8,9].
2. Chitin Sources and Obtaining Methods and Applications
- α-chitin is the most abundant and stable polymorph which is more common than the β-type, it has an 80% crystallinity index [10]. Arrangement of microfibrils in a-chitin is strongly fixed by hydrogen bonds that limits its water swelling and permeability compared with p-chitin with a lower content of inter-sheet hydrogen bonds. Also, the proportion of chitin in the source material (such as the raw shells) in-fluences the hardness, permeability, and flexibility [11]. Large available quantity of shrimp and crab shells makes them the most popular source of the white, hard, inelastic, nitrogenous polysaccharide, chitin. The main sources of raw material for chitin ptoduction on commercial scale are crustacean exoskeletons, principally crabs and shrimps. And other sources insect shells.
2.1. Obtaining Methods Chitin/Chitosan
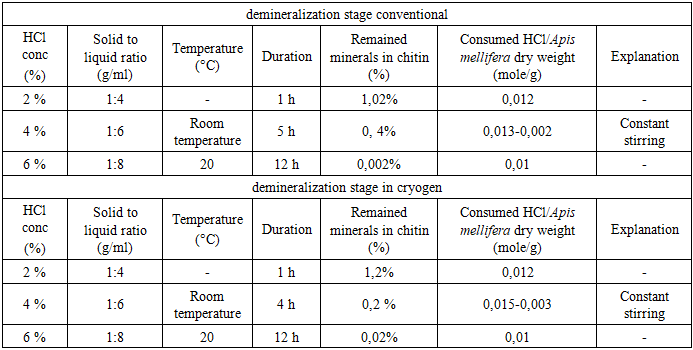
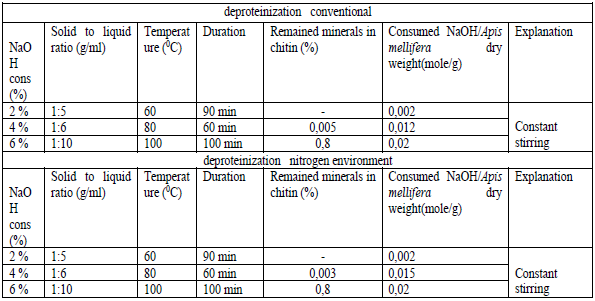
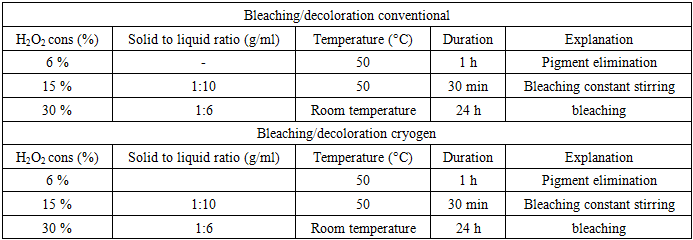
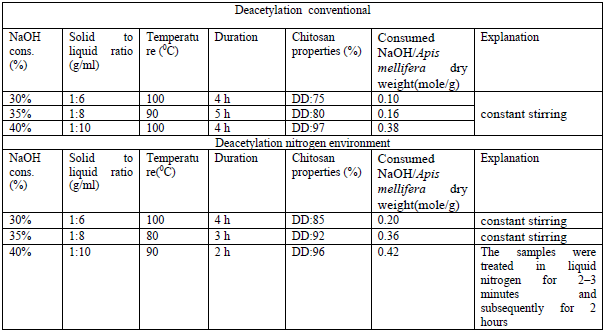
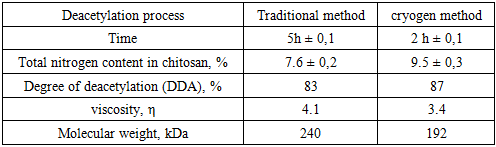
- The honey bees contain three major components; 25-35% a-chitin, 40-50 % proteins, 5-10% lipid and 2-5% CaCO3. Pigments and other metal salts are also the minor components [12,13,14]. The chemical isolation process is composed of four steps: removal of inorganic CaCO3 (demineralization), elimination of proteins (deproteinization), removing small amounts of pigments (decoloration/bleaching), and converting chitin to chitosan (deacetylation). Some studies have changed the arrangement of these steps [15]. Also, some pre-treatments such as grinding for enhancing of solution exposure and few post-treatments like purification techniques may be added for increasing the quality of the final product. The process of obtaining chitosan consists of several stages, starting with the collection of seasonally dead honeybees, followed by washing and drying. The cleaned and dried bees were then subjected to stepwise processing under both conventional methods and liquid nitrogen conditions. For both approaches, the demineralization stage was carried out using three different concentrations, after which the samples were dried. The results of the dry mass were compared [16] (table 1).
|
|
|
|
|
2.2. Chitin /Chitosan Applications
- Chitosan functions as a natural biopolymer enhancing plant growth and crop protection. It stimulates seed germination, root development, and stress resistance; acts as a biopesticide and fungicide against fungi, bacteria, and nematodes; improves soil microbial activity and nutrient retention as a soil conditioner; and enhances seed performance when used as a coating. Due to its biocompatibility and hemostatic properties, chitosan accelerates wound healing and is used in surgical dressings. It serves in drug-delivery systems (nanoparticles, hydrogels, films) enabling controlled release, and as a scaffold in tissue engineering for bone, cartilage, and nerve regeneration. Its antimicrobial activity and ability to bind dietary fats and cholesterol support applications in infection control and metabolic regulation. Food Industry-chitosan extends food shelf life through its antimicrobial and antioxidant properties. It is employed as an edible coating on fruits, vegetables, and seafood to reduce spoilage, as a dietary fiber supplement with lipid-binding capacity, and in biodegradable food-packaging films as a sustainable alternative to plastics. Chitosan efficiently adsorbs heavy metals, dyes, oils, and phosphates in wastewater treatment, is utilized in air-filtration materials for toxin removal, forms biodegradable composites replacing synthetic polymers, and has strong hydrocarbon adsorption capacity useful in oil-spill cleanup. In textiles, chitosan improves dye uptake in cotton, wool, and polyester blends; provides durable antimicrobial finishing preventing bacterial and fungal growth; acts as an antistatic and softening agent enhancing comfort; and supports development of medical textiles such as bandages and hospital gowns.As a film-forming moisturizer, chitosan enhances skin hydration; strengthens and protects hair, improving gloss and reducing breakage; and exhibits antibacterial activity in toothpaste and mouthwash formulations.Chitosan is applied in enzyme and cell immobilization for biocatalytic processes, in the production of biodegradable bioplastics, as an eco-friendly component in agricultural sprays and coatings, and in nanotechnology for targeted drug delivery and biosensor design.
 | Figure 1. Chitin/chitosan based biomaterial applications [21] |
3. Physicochemical Properties Chitosan
- The above figure 2 presents the scanning electron microscopic (SEM) image and elemental analysis of chitosan extracted from Apis mellifera (dead honey bees) obtained via the conventional method.
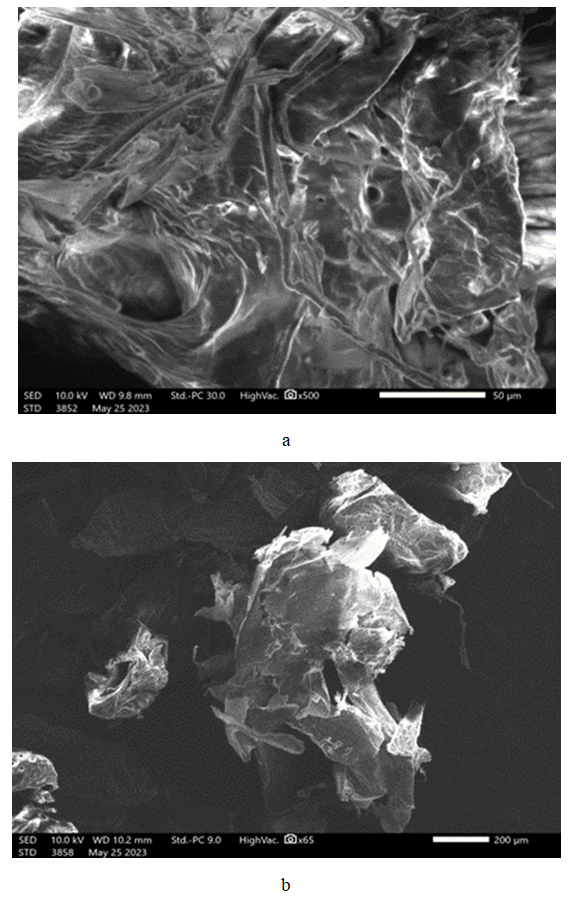 | Figure 2. SEM analysis chitosan: a) traditional b) cryogen |
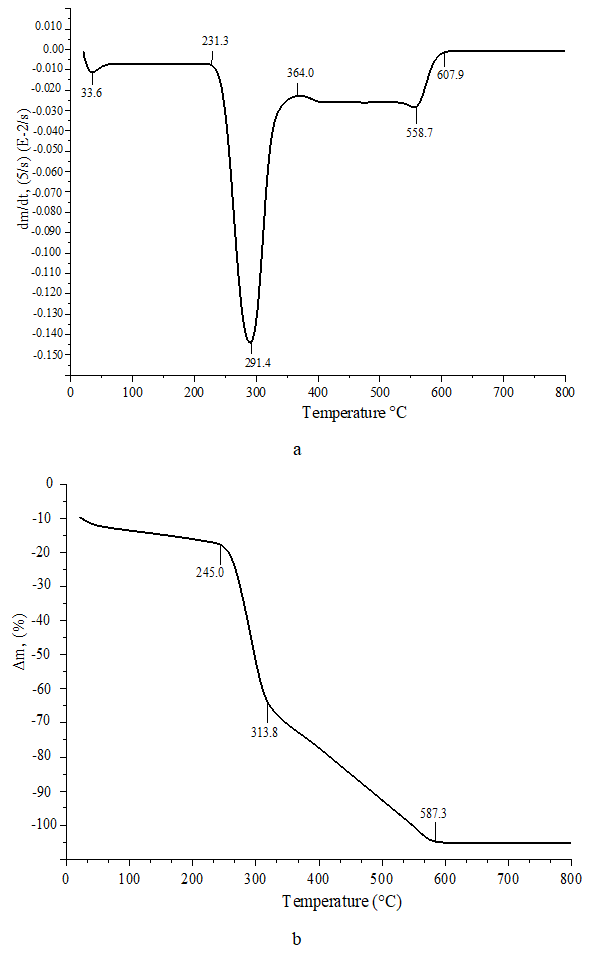 | Figure 3. a) DTG b) TG analysis chitosan Apis mellifera |
4. Using Dyeing Process and Environmental Impacts
- Chitosan friendly cationization agent. Chitosan increases dye uptake in cotton and polyester–cotton blends by providing additional binding sites. In additional, salt-free dyeing: helps avoid the use of large amounts of electrolytes, making dyeing more eco-friendly and color fastness: improves wash fastness and fixation of reactive, acid, disperse and natural dyes [22]. Chitosan is a biodegradable, renewable, and non-toxic biopolymer derived mainly from chitin found in crustacean shells and insect biomass. Its production and application offer significant environmental advantages compared to petroleum-based polymers. During production, chitosan synthesis involves deproteinization, demineralization, and deacetylation using alkaline and acidic reagents. Although these steps generate some chemical effluents, modern methods - such as microwave-assisted, enzymatic or cryogenic processing-have reduced reagent consumption, energy use and wastewater discharge. From an ecological perspective, chitosan contributes to sustainable development due to its biodegradability and ability to replace synthetic polymers in packaging, textiles, water treatment, and agriculture. It decomposes naturally into harmless products such as carbon dioxide, water, and nitrogen compounds, minimizing environmental persistence. Moreover, chitosan’s ability to adsorb heavy metals, dyes, and pollutants makes it an effective material for environmental remediation, particularly in wastewater treatment. When produced from waste biomass such as shrimp shells or dead honey bees, chitosan also supports waste valorization and circular economy principles.
5. Conclusions
- In the research work, chitosan was extracted from local secondary raw materials using two different methods, and the kinetics of the extraction processes were compared. The physicochemical properties of the obtained chitosan samples were analyzed. This biopolymer, which has a wide range of applications, was utilized in the dyeing process of mixed fabrics in the textile industry. Overall, chitosan represents a green alternative polymer that reduces ecological footprint, promotes waste recovery, and supports eco-friendly industrial innovation.
ACKNOWLEDGEMENTS
- The authors would like to express their sincere gratitude to the Department of General Chemistry, Tashkent State University named after Islam Karimov, for their support during the preparation of this research. Special thanks are extended to the scientific supervisor, Ikhtiyarova G.A, for valuable guidance, constructive feedback, and continuous encouragement throughout the study.
Declaration
- The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML