-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Polymer Science
p-ISSN: 2163-1344 e-ISSN: 2163-1352
2025; 14(2): 30-38
doi:10.5923/j.ajps.20251402.03
Received: Aug. 19, 2025; Accepted: Sep. 5, 2025; Published: Sep. 8, 2025

Molecular Structure and Potential of Silk Fibroin as a Biomaterial: A Review
Shigabutdinov A. A.1, Eshchanov Kh. O.2, Baltayeva M. M.2
1PhD Student in the Department of Chemistry, Urgench State University named after Abu Rayhan Beruni, Uzbekistan
2Chemistry Department Lecturer, Urgench State University named after Abu Rayhan Beruni, Uzbekistan
Correspondence to: Shigabutdinov A. A., PhD Student in the Department of Chemistry, Urgench State University named after Abu Rayhan Beruni, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Silk fibroin (SF), a natural fibrous protein produced by the Bombyx mori silkworm, exhibits a unique combination of high tensile strength, biocompatibility, biodegradability, and tunable structural properties, making it an attractive candidate for advanced biomaterial applications. This review presents a comprehensive examination of SF’s molecular structure, from its amino acid sequence and β-sheet crystalline domains to its amorphous regions and hierarchical fiber organization, elucidating how these features dictate its mechanical, thermal, hygroscopic, and biological behavior. Recent progress in SF modification—encompassing chemical functionalization, enzymatic processing, and physical treatments such as plasma activation—has expanded its applicability, enabling the fabrication of nanocomposites with enhanced antimicrobial, conductive, and bioactive properties. Applications in tissue engineering, wound healing, implantable devices, flexible bioelectronics, and environmental remediation are critically discussed. Key challenges remain, including incomplete understanding of long-term degradation pathways, safety standards for nano-additives, and industrial scalability of advanced processing techniques. Addressing these limitations will be crucial for realizing SF’s full potential as a sustainable, multifunctional biomaterial for regenerative medicine, biotechnology, and eco-friendly industrial solutions.
Keywords: Biomaterial, Silk, Fibroin, Molecular structure, Modification, Nanocomposites
Cite this paper: Shigabutdinov A. A., Eshchanov Kh. O., Baltayeva M. M., Molecular Structure and Potential of Silk Fibroin as a Biomaterial: A Review, American Journal of Polymer Science, Vol. 14 No. 2, 2025, pp. 30-38. doi: 10.5923/j.ajps.20251402.03.
Article Outline
1. Introduction
- Fibrous proteins such as silk, collagen, keratin, and elastin are widely distributed in various biological tissues. Their primary distinction from other proteins lies in their fibrillar nature. These proteins typically possess a highly ordered secondary structure and are built upon repetitive amino acid sequences. Most fibrous proteins lack complex tertiary and quaternary structures; instead, they form highly organized nanofibrils and microfibrils, which differentiates them from globular (spherical) proteins. These nanofibrils and microfibrils, in turn, assemble into complex, ordered structures in one, two, or three dimensions [1].Fibrous proteins are of significant importance primarily due to their wide availability and renewable nature. For instance, silk has been used as a textile fiber for thousands of years. However, in recent years, they have been recognized as promising materials because of their unique biological properties, such as biodegradability, applicability in their natural form, strength, softness, smoothness, chemical modifiability, and the ability to be disassembled and reassembled under artificial conditions [1].Environmental issues—such as pollution, ozone layer depletion, loss of biodiversity, and the destruction of natural habitats—are largely the result of excessive human exploitation. In particular, the improper use of polymer materials poses a significant ecological threat. Interestingly, while nature itself has been harmed, it also offers solutions to these problems [2].Silk is produced for various biological purposes: for example, silkworms spin cocoons to protect themselves from external factors, while spiders spin webs for hunting. Some insects also produce silk during their reproductive cycle. Despite structural differences, all types of silk exhibit a high degree of crystallinity and have a similar amino acid composition.Silk is a widely used natural biopolymer, and thanks to advances in artificial silkworm rearing and breeding technologies, fibroin can be obtained in large quantities (Figure 1). Although spider silk has superior quality characteristics, it cannot be produced on an industrial scale. In recent years, technological and genetic modifications of silk fibroin have significantly improved the quality parameters of silkworm fibroin.
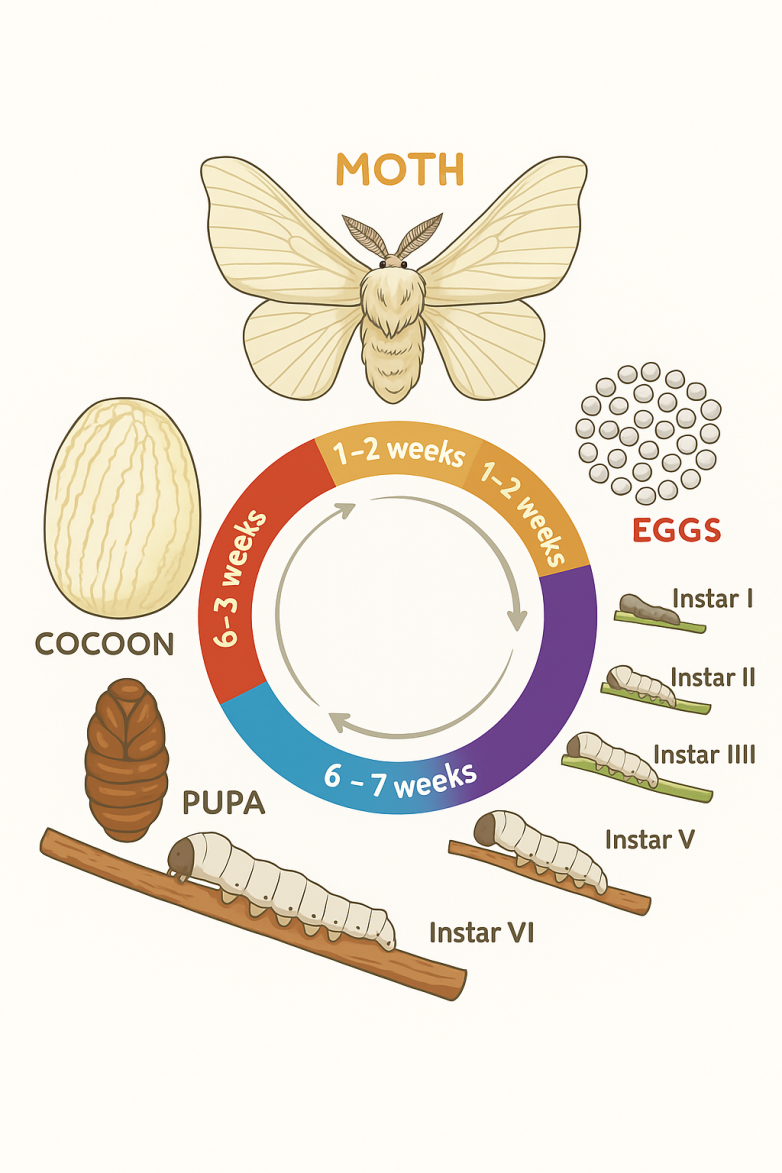 | Figure 1. Schematic of the life cycle of the Bombyx mori silkworm |
2. Silk Fibroin: Molecular Structure, Physicochemical Characteristics, and Biomedical Applications
- The structure of the Bombyx mori silkworm cocoon consists of a single filament measuring between 700 and 1500 meters in length and having a diameter of 10–20 micrometers (Figure 2). Each filament is composed of bundles of fibrils with diameters of 20–30 nanometers. These fibrils are intertwined with one another, and, much like a rope whose strength derives from the twisting of smaller strands, this arrangement enhances the overall tensile strength of the silk fiber [4], [5].
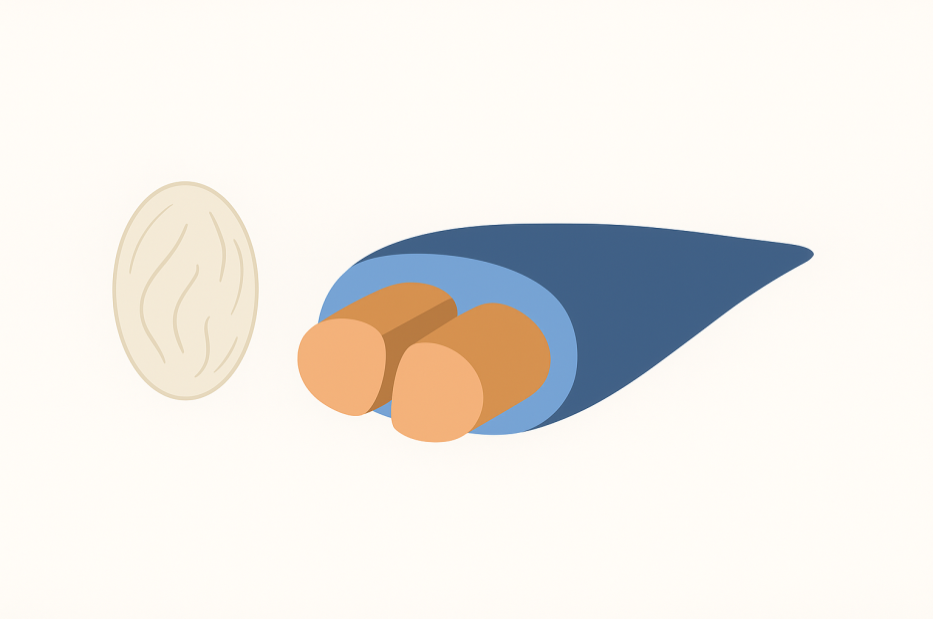 | Figure 2. Graphical depiction of Bombyx mori cocoons, showing the arrangement of their main components - fibroin and sericin - in forming the fibers that make up the cocoon |
2.1. Molecular Composition of Silk Fibroin
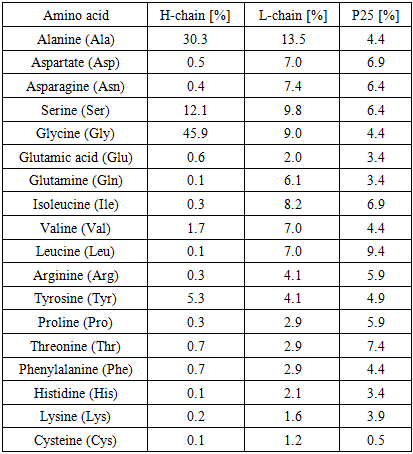
- Silk fibroin is composed of heavy (H, ~390 kDa) and light (L, ~26 kDa) chains, which are linked together at the C-terminal of the H-chain by a single disulfide bond to form an H–L complex. This H–L complex is further associated, through non-covalent interactions, with a P25 glycoprotein of approximately 25 kDa molecular weight. Silk fibroin consists of these three components in a molar ratio of 6:6:1.The amino acid composition of Bombyx mori silk fibroin is predominantly glycine (43%), alanine (30%), and serine (12%). The hydrophobic domains of the H-chain are made up of repeating Gly-Ala-Gly-Ala-Gly-Ser (Figure 3) hexapeptide sequences and Gly-Ala/Ser/Tyr dipeptide repeats, which can form stable antiparallel β-sheets. In contrast, the amino acid sequence of the L-chain is non-repetitive, relatively hydrophilic, and exhibits a more elastic structure [8], [9], [10].
2.2. Structure of Silk Fibroin
2.2.1. Primary Structure
- In numerous previous studies, it has been suggested that the primary structure of Bombyx mori fibroin consists of simple and regular repeating units, with approximately 70% of its amino acid sequence represented by the motif (Gly-Ser-Gly-Ala-Gly-Ala-)ₙ [11] (Figure 3). Currently, it is known that the H-fibroin chain is composed of highly repetitive crystalline fractions, with these repeating sequences typically in the form of Gly–X. Here, the ‘X’ position is predominantly occupied by alanine (Ala) – 64%, serine (Ser) – 22%, tyrosine (Tyr) – 10%, valine (Val) – 3%, and threonine (Thr) – 1.3%. The hydrophobic domains of the H-fibroin chain form antiparallel β-sheets through hydrogen bonding and hydrophobic interactions. These structural units are the key contributors to the high tensile strength of silk fibroin [12], [13].
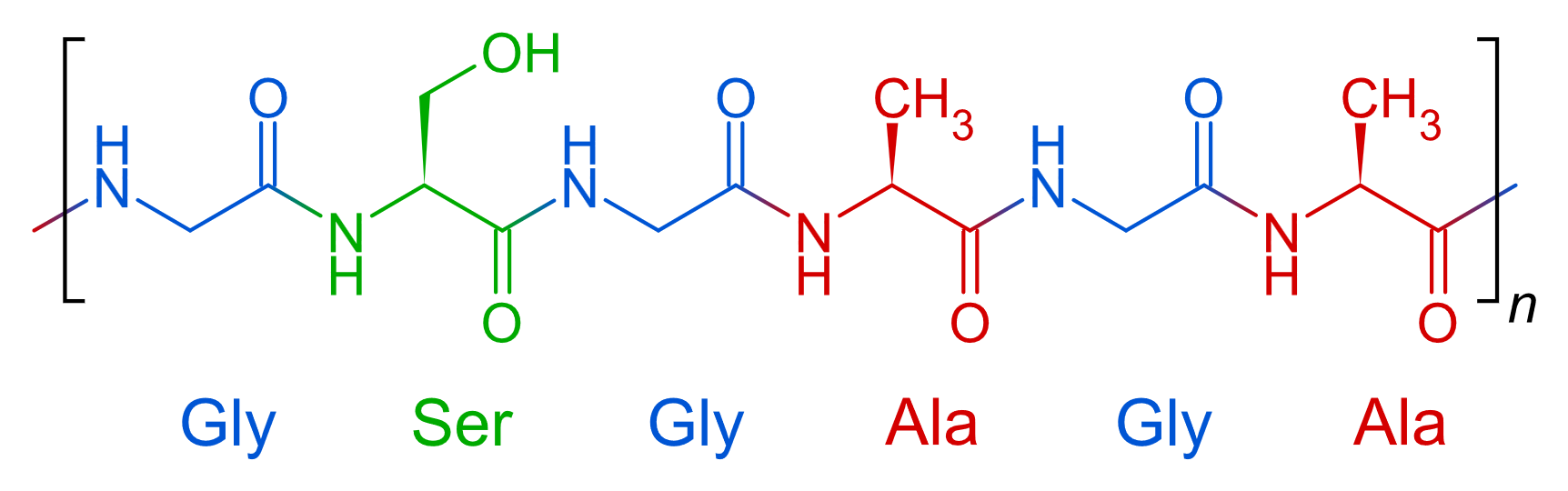 | Figure 3. Primary Structure of Silk Fibroin |
|
2.2.2. Secondary Structure
- The primary structure of silk fibroin is described as a simple and regular unit. However, its higher-order structures are relatively complex and remain one of the debated topics in scientific circles. At present, this complex architecture is regarded as one of the main focuses of silk research.In general, fibrous proteins, due to their highly repetitive amino acid sequences, possess either a dominant single secondary structure or a “block” architecture. Each of these blocks forms a specific conformational structure. Moreover, these repetitive sequences allow the proteins to adopt regular conformations that are otherwise rare.Many fibrous proteins consist of two types of repeating blocks with distinct chemical properties: crystalline (hydrophobic) blocks and amorphous (hydrophilic) blocks.Crystalline blocks are usually composed of short side chains, highly repetitive amino acids, which result in the formation of antiparallel β-sheets. Non-crystalline regions are often poorly ordered, twisted ribbon-like, or amorphous. Many silk types, including Bombyx mori fibroin, exhibit polymorphism—meaning their predominant secondary structure can change depending on environmental conditions. The conformational flexibility provided by glycine allows silk to transition from a water-soluble form to an insoluble β-sheet structure.Fibroin has two crystalline polymorphic forms, referred to as silk I and silk II. Silk I is the form produced naturally. This state is metastable, thermodynamically unstable, and water-soluble. It is amorphous, hydrophilic, and typically exhibits an α-helix or twisted ribbon structure. Silk II, on the other hand, is thermally stable, crystalline, and primarily composed of β-sheets. It is hydrophobic, insoluble, and possesses strong mechanical properties. Under external stimuli (e.g., changes in pH, temperature, stretching, or alcohol treatment), β-sheet formation can drive the transformation from silk I to silk II. [16], [17], [18], [19], [20].The density of silk I is 1.40 g/cm³, while that of silk II is 1.45 g/cm³. In practical silk fibroin samples, however, the density typically ranges between 1.31 and 1.325 g/cm³, which can be attributed to the ratio of crystalline to amorphous regions.Silk III is a relatively rare form characterized by a helical crystalline structure, found primarily in thin films prepared at the water–air interface. Silk III is not the main crystalline structure of silk fibroin and is considered a relatively rare form. In most cases, fibroin molecules adopt β-sheet conformations because they are thermodynamically stable and energetically favorable under normal conditions. By contrast, Silk III is metastable, since it can only be formed under specific conditions, such as exposure to certain solvents, particular pH environments, or at the air–water interface. One of the most distinctive features of silk III is its left-handed helical conformation, in which hydrophilic serine and hydrophobic alanine residues are positioned on opposite sides of the chain. This arrangement enables the fibroin molecule to exhibit surface-active properties [21].The ratio of crystalline to amorphous domains, the interaction of structures with water (hydrophilicity or hydrophobicity), the density and type of crystalline nodes (primarily silk I or silk II phases), as well as their size, distribution, and orientation—all these factors exert a strong influence on the final properties of silk fibroin. This semi-crystalline state is fully tunable, meaning that each structural component can be adjusted to achieve specific properties.Strong lateral interactions occur between nanofibrils, preventing their relative sliding. As a result, when silk fibers undergo deformation, the propagation of cracks is inhibited. This behavior enhances the strength of the silk fibers and improves the overall mechanical durability of the structure [22].
2.3. Physicochemical Properties
2.3.1. Mechanical Properties
- The cocoon protects the silkworm from mechanical damage, and as a result, silk fibroin possesses unique mechanical characteristics. Among natural fibers, silk is one of the lightest, yet its tensile strength surpasses that of many biomaterials (such as cotton and polylactic acid (PLA)). Silk fiber is composed mainly of low-density chains and microfibrils, making it one of the lightest fibers. In silk fibroin, the β-sheet crystal domains have a strong structure, forming extensive hydrogen bonds, which provide mechanical strength. At the same time, the β-sheet-shaped amorphous domains provide electrical energy and various impacts absorb energy, protecting the fiber from breakage. This determines its tensile strength. The restructuring of the lightweight, high degree of crystallinity of the domains, and the efficient distribution of force between the crystalline and amorphous domains make silk stronger than many natural and synthetic biomaterials. Remarkably, it is even stronger than a steel wire of equivalent diameter [23].Natural silk fibroin (SF) fibers are characterized by distinctive mechanical properties, including a maximum elongation of 13–14%, an elastic modulus in the range of 15–18 GPa, and a tensile strength between 620 and 760 MPa.These properties remain almost unchanged across different cocoons. [24] Silk fibroin demonstrates an unusual combination of strength and toughness, along with an excellent energy absorption capacity, which distinguishes it from other natural and synthetic fibers. These properties are comparable to those of certain high-performance fibers, such as nylon. Its composition and molecular structure primarily determine the mechanical behavior of SF within the fibers [25], [26].
2.3.2. Hygroscopic Properties
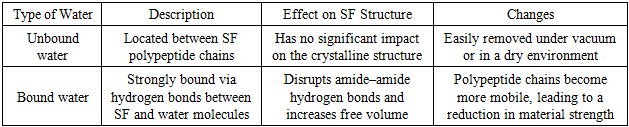
- The presence of polar amino acid residues in silk fibroin (SF) renders it a hygroscopic material, capable of absorbing moisture. Water is primarily retained within the amorphous regions, as the dense packing of the crystalline domains hinders the penetration of water molecules. Consequently, in natural silk fibroin, a higher degree of crystallinity corresponds to a lower water content.However, processing of SF allows control over its water content [27]. The absorbed water may exist in two forms (Table 2):
|
2.3.3. Thermal Properties
- During metamorphosis, silk fibroin (SF) serves as a thermal insulator, protecting the cocoon. The thermal diffusivity coefficient (α) of SF ranges from 1 × 10⁻⁷ m²/s to 1.6 × 10⁻⁷ m²/s. Interestingly, this value decreases with an increasing content of β-sheet structures [28], [29].The water content and degree of crystallinity in SF strongly influence its thermal transitions (thermal phases). In water-saturated SF, the first thermal transition occurs between −60°C and 0°C, which is associated with hydrogen bonding between SF and water molecules. At around 60°C, SF exhibits a second endothermic transition, typically attributed to the increased mobility of amorphous domains or the disruption of protein–water interactions. As a result, molecular motions intensify, and the plasticity of SF materials increases — this stage is referred to as the low-temperature glass transition [30], [31], [32].At approximately 100°C, unbound (free) water begins to evaporate, while at 130°C, α-helical conformations gain mobility. Around 140°C, hydrogen bonds within the α-helices are disrupted. Hydrogen bonding is a weak and non-covalent bond, and at high temperatures, this bond is broken due to the energy required, resulting in the ordered α-helical conformation in production losing its protection and partially unfolding. This process leads to a change in the arrangement and transition to a disordered or β-sheet conformation, and changes the mechanical and thermal properties of the silk fiber.Between 160–170°C, bound water is completely released, leading to a glass transition of SF [33], [34], [35]. Above the glass transition temperature, but before reaching 210–214°C, the chains released from α-helices and the mobile amorphous domains undergo recrystallization, forming β-crystals - a process that stabilizes the entire structure. SF remains thermally stable up to 280–290°C. Beyond this temperature, degradation begins due to cleavage of side chains, amino acid residues, and peptide bonds in the polypeptide chains. The second degradation stage occurs at approximately 325°C, where thermal decomposition of peptides results in around 50% mass loss [23], [36], [37].At temperatures above 700°C, almost no residue remains due to the very low inorganic content of SF.
2.3.4. Biocompatibility and Biodegradation
- Silk fibroin is a naturally occurring, highly purified protein synthesized and secreted by the cells lining the silk gland of the silkworm. It is well tolerated by the body, which can metabolize the degradation products. The molecular weight of silk fibroin can be tailored by modifying its composition, enabling adaptation to the requirements of various biological environments [38]. Consequently, silk fibroin exhibits excellent biocompatibility.Biodegradation of silk fibroin occurs through enzymatic (e.g., protease-mediated) or hydrolysis-based mechanisms. The degradation rate depends on factors such as the degree of crystallinity, β-sheet content, processing method, pH, temperature, and enzyme concentration [39].As one of the oldest natural fibers, silk has been used as a surgical suture material for centuries. Currently, when silk fibroin is applied as a drug delivery carrier, its biocompatibility and safety remain the primary considerations. When silk fibroin is used as a drug carrier, it interacts directly with living tissues and cells in the body, so its safety and biocompatibility are very, very important factors. Because any negative effects on the body, such as cytotoxicity or the formation of harmful products as a result of its decomposition and accumulation in the body, can weaken the patient's immune system, as well as reduce the effectiveness of treatment. Since silk fibroin is a natural protein, it breaks down into non-toxic amino acids in the body, does not negatively affect the immune system and acts as a stable protective shell for drug molecules. Therefore, its safety and biocompatibility are important factors [40], [41]. In a study where silk-based stents were implanted into mice, virtually no inflammatory response was observed after one year of implantation [42].Wang and colleagues applied a vacuum–ultraviolet–ozone activation method to coat Mg/Zn/Ca alloy surfaces with a protective layer of silk fibroin, followed by in vitro and in vivo implantation in cell cultures and rabbits. The results demonstrated enhanced corrosion resistance of the alloy and improved compatibility with bone marrow-derived stem cells [43].Other researchers have evaluated the safety and efficacy of silk fibroin microparticle scaffolds for filling bone cavities [44]. Furthermore, the safety and performance of tissue-engineered constructs composed of silk fibroin and collagen have been tested in patients with bone defects. The results confirmed that the materials were both safe and effective, recommending larger-scale controlled clinical studies as the next step [45].Collectively, these findings highlight silk fibroin as a unique natural polymer distinguished by its high biocompatibility and biological safety, offering significant scientific and practical potential for drug delivery applications. Its natural origin, adaptability to diverse biological environments, and ability to integrate directly with cells and tissues make it a promising material for modern biomedicine and pharmaceutical sciences.
2.4. Recent Advances
- Silk fibroin (SF), like many biopolymers, has recently gained significant attention in fundamental and materials science research due to its “green” nature. This aligns with the principles of sustainable development and significantly influences modern industrial processing trends. Innovative solutions based on biodegradable and biocompatible natural materials are currently being effectively applied across various fields, including packaging, medicine, food technology, and agriculture.A key advantage of SF-based materials lies in their ability to be processed from an amorphous aqueous solution as the starting feedstock. This approach enables the fabrication of materials with diverse mechanical properties, tailored to specific regenerative processes, while maintaining the physiological adaptability of SF materials [46], [47].The biocompatibility of SF-based materials is determined by several criteria, including their interaction with biological systems, the level of immunogenicity reflected in a low or negligible immune response, mechanical properties such as elasticity, strength, and flexibility, the nature of degradation products that should be non-toxic to the organism, and their ability to integrate with surrounding tissues during the regeneration process.Controlling the regeneration process of SF is possible by modifying its crystallinity, hydrophobicity, material form, and mechanical properties. These characteristics have driven the development of advanced biomatrix fabrication technologies.The physicochemical and mechanical properties of SF enable its application across multiple domains. In the biomedical field, SF-based scaffolds serve as three-dimensional biomatrices that support cell growth and tissue regeneration, and they are extensively employed in wound dressings, artificial ligaments, and bone tissue engineering. These structures provide an optimal microenvironment for cell adhesion, proliferation, and differentiation, while maintaining both biocompatibility and biodegradability [48], [49]. In electronics, SF-based materials function as advanced biomaterials for the development of biocompatible and environmentally benign sensors and electronic devices. Their transparency, dielectric properties, and mechanical flexibility make SF an excellent platform for biosensors that detect biological signals, implantable electronic systems, and light-managing optical components [50], [51]. In environmental applications, SF-based materials exhibit natural biodegradability, non-toxicity, and a high sorption capacity, rendering them highly effective in biofiltration systems and waste treatment technologies. Their large surface area, chemical modifiability, and strong ability to adsorb heavy metal ions and organic pollutants position SF materials as promising candidates for industrial wastewater purification, air filtration, and hazardous waste neutralization, with SF-based biofilters operating under minimal environmental impact [52], [53], [54].These advantages enable SF-based materials to be produced with high efficiency and low environmental impact. The extraction of silk fibroin (Bombyx mori) involves relatively simple processes. Silk can be purified from sericin, degummed, and dissolved using mild solvents. This requires less energy than synthetic polymer production. Also, environmentally friendly methods are used to process and recycle fibroin. For example, working in an aqueous solution, freeze-drying, and electrospinning. Therefore, the environmental impact is low, the production process is efficient, inexpensive, and waste-free.Silk fibroin, as a protein-based natural biopolymer, can undergo various chemical, physical, and biological modifications, each of which significantly broadens its functional versatility and expands the scope of its potential applications. Chemical modification involves treatment with agents such as thiourea, glutaraldehyde, persulfates, anhydrides, silanes, or polyelectrolytes, which not only enhance the hydrophilic or hydrophobic properties of the fibroin surface but also enable the introduction of additional functional groups that can improve its reactivity and compatibility with diverse environments [54], [55]. Physical modification, on the other hand, is primarily directed toward altering the surface energy and generating new reactive sites by means of techniques such as ultraviolet (UV) irradiation, plasma treatment, or ozone oxidation, thereby creating surfaces that are more favorable for subsequent chemical interactions or biological responses [56], [57]. In addition, biological modification, most commonly achieved through enzymatic treatment—for example, by employing lipase—enables the restructuring of fibroin chains or the introduction of specific active groups, which provides a more biocompatible and selective approach to tailoring its physicochemical characteristics for biomedical and environmental applications [58].These processes enhance cell adhesion, protein interactions, sorption properties, and mechanical durability of SF materials.SF-based nanocomposites represent a new generation of materials formed by incorporating nanomaterials (e.g., nanogels, graphene oxide, carbon nanotubes, nanoparticles) into the SF matrix. Their goal is to preserve the inherent advantages of fibroin while adding new functionalities. The functional performance of silk fibroin-based materials can be further enhanced through the incorporation of different nanomaterials, each imparting distinct properties that expand their application potential. The integration of metal nanoparticles such as Ag, Au, ZnO, and TiO2 provides antibacterial activity and photocatalytic functionality, which are highly valuable in biomedical applications and environmental remediation [59]. Similarly, the incorporation of carbon nanostructures, particularly graphene oxide, significantly improves the electrical conductivity of silk fibroin composites, thereby enabling their utilization in flexible electronic devices and biosensors designed for sensitive biological signal detection [60]. In addition, ceramic nanomaterials such as silicon dioxide contribute to the acceleration of biomineralization processes, making them particularly effective in bone and dental tissue regeneration, where controlled mineral deposition is essential for structural and functional recovery [49].Nanocomposites exhibit increased surface area, strength, thermal stability, and chemical resistance, making them highly versatile for applications in environmental protection, biomedicine, electronics, and the food industry.
3. Conclusions
- Recent research has demonstrated that silk fibroin (SF) and regenerated fibroin (RF) materials possess exceptional scientific and technological potential as highly biocompatible, biodegradable, and mechanically adaptable biomaterials. RF-based materials have been successfully tested in tissue engineering, wound dressing systems, artificial implants, bone regeneration, biocompatible sensors, electronic devices, and environmental biofiltration systems.Various modification techniques — chemical (crosslinking, introduction of functional groups), biological (enzymatic treatment), and physicochemical (plasma treatment, UV irradiation) — have enabled improvements in surface properties, enhancement of mechanical strength, and efficient immobilization of biologically active molecules. Through nanocomposite technologies, RF has been integrated with nanohydroxyapatite, silver, gold, TiO₂, ZnO, and other nanomaterials, significantly enhancing its antimicrobial and electroactive properties. Additionally, the use of 3D bioprinting and electrospinning technologies has expanded the ability to create scaffolds with complex geometries, tailored for cell integration and tissue regeneration.However, literature analysis reveals several limitations. The long-term degradation behavior of processed fibroin and the effects of its degradation products on the body remain insufficiently studied. There are no unified safety standards for metallic and metal oxide nanoparticles used in nanocomposites. Moreover, the effects of various technologies (e.g., 3D bioprinting, electrospinning) on the structure and biological properties of fibroin are not yet fully understood. In addition, when plasma treatment and other surface modification techniques are implemented on an industrial scale, issues of energy efficiency, economic feasibility, and technological sustainability have not been comprehensively addressed.Silk fibroin, with its unique mechanical, biological, and tunable functional properties, is recognized as a next-generation biopolymer of strategic importance for the creation of smart biomaterials, regenerative medicine, bioengineering, and advanced biotechnologies.Fibroin — the Biopolymer of the Future.
Declaration
- The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
ACKNOWLEDGEMENTS
- The authors would like to express their sincere gratitude to the Department of Chemistry, Urgench State University named after Abu Rayhan Beruni, for their support during the preparation of this research. Special thanks are extended to the scientific supervisor, Baltayeva M.M., for valuable guidance, constructive feedback, and continuous encouragement throughout the study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML