-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Polymer Science
p-ISSN: 2163-1344 e-ISSN: 2163-1352
2024; 13(1): 1-5
doi:10.5923/j.ajps.20241301.01
Received: Jan. 30, 2024; Accepted: Feb. 20, 2024; Published: Feb. 24, 2024

Influence of Chemical and Physical Refining Processes on Phospholipid and Total Tocopherol Content in Sunflower Oil
Оybek Zufarov1, Kamar Serkayev2, Shoirakhon Isroilova3
1Association of Fat and Oil Industry Enterprises of Republic of Uzbekistan, Tashkent, Uzbekistan
2Doctoral Student, Department of Food Technology, Tashkent Institute of Chemical Technology, Tashkent, Uzbekistan
3Doctor of Technical Sciences, Department of Food Technology, Tashkent Institute of Chemical Technology, Tashkent, Uzbekistan
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

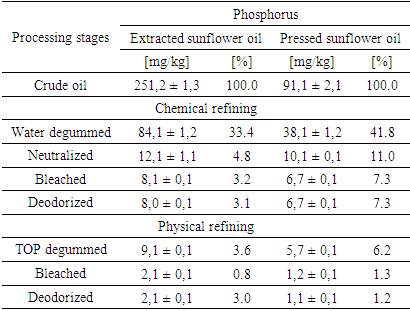
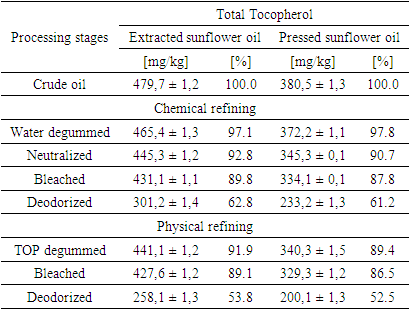
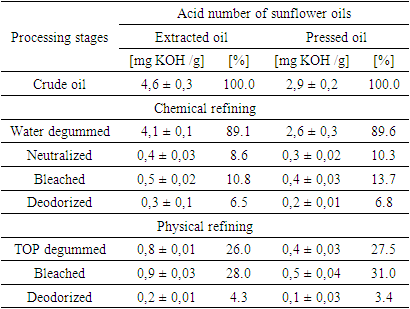
Currently, two major methods of oil refining are widely used: chemical and physical refining. This study compared the effects of these processes, including degumming, bleaching, neutralization, and deodorization, on the removal of phosphorus, free fatty acids, and the loss of tocopherols in sunflower oil. The efficiency of phosphorus removal, acid value reduction, and the impact of these methods on tocopherol losses in oils were assessed.The results indicated that physical refining is the most effective method for oil refining but has a negative impact on tocopherol content in the oils. Each refining stage had a distinct influence on phosphorus removal, acid value, and tocopherol losses in the oil. In physical refining, phosphorus content in the oils decreased by less than 2.1 mg/kg, while in chemical refining, it decreased by less than 8.0 mg/kg. Tocopherol content after physical refining was 51%, compared to 61% in chemical refining. Tocopherol loss during physical refining was higher by 10% than in chemical refining due to the high temperatures used during the deodorization process, which removes free fatty acids in sunflower oil. In chemical refining process, sunflower oil retained more tocopherols compared to the physical refining method. However chemical refining is considered less efficient due to saponification of neutral oil.
Keywords: Sunflower oil, Chemical refining, Physical refining
Cite this paper: Оybek Zufarov, Kamar Serkayev, Shoirakhon Isroilova, Influence of Chemical and Physical Refining Processes on Phospholipid and Total Tocopherol Content in Sunflower Oil, American Journal of Polymer Science, Vol. 13 No. 1, 2024, pp. 1-5. doi: 10.5923/j.ajps.20241301.01.
1. Introduction
- Vegetable oils are obtained through two primary methods: pressing and extraction. Oils obtained by these methods are considered crude oils and contain not only triacylglycerides but also various accompanying substances such as phospholipids, pigments, tocopherols, waxes, non-saponifiable matter, oxidative products, and others. The quantities of these accompanying substances depend on the oil extraction method, with extraction oils containing more of these substances compared to oils obtained by pressing. This is because during extraction with solvents, a significant portion of these accompanying substances is transferred into the oil. Some of these accompanying substances, such as diglycerides, vitamins, phytosterols, tocopherols, and polyphenols, have important health benefits [1,2], and therefore, they should not be removed during oil refinement. On the other hand, other compounds known for their negative impact on oil quality and stability include free fatty acids, non-saponifiable matter, waxes, pigments, and oxidation products. Their presence in oils is undesirable as they can affect stability and sensory acceptability for consumers [3,4]. The refinement of vegetable oils can be categorized into two main methods: chemical and physical refining [5].Chemical refining is an older method and consists of several stages, with each stage having specific functions for removing certain undesirable compounds. The stages of chemical refining include degumming to remove phospholipids [6], neutralization, where free fatty acids are removed through caustic soda neutralization [7] (the use of caustic soda for oil refinement was first employed in 1842 with cottonseed oil [8], and its industrial use in refining vegetable oils began in 1932 [9]. The quantity and concentration of aqueous caustic soda solutions used in oil refinement depend on the type of oil. Bleaching is aimed at removing pigments [10,11], and the final stage of chemical purification is deodorization, which allows the removal of volatile substances, carotenoids, and free fatty acids [12-14]. However, chemical refining has several disadvantages as certain biologically active molecules are also removed at each stage of the process. These molecules mainly consist of tocopherols and polyphenols, which can act as antioxidants [15,16].Physical refining is a relatively newer method of oil refinement and consists of three main processes: hydration, bleaching, and deodorization. The key difference between chemical and physical refining lies in the removal of free fatty acids. In chemical refining, this is achieved by adding caustic soda and separating the soap through centrifugation. In contrast, physical refining removes free fatty acids and other compounds during the deodorization process. Physical refining was first applied in 1930 and used on an industrial scale in 1950 during the refinement of palm oil, which had relatively low phospholipid content and high free fatty acid content [17]. The efficiency of phosphorus removal in vegetable oils is a critical factor in physical refining [18].In crude vegetable oils, phospholipids exist in both hydrated forms (HPL) and non-hydrated forms (NHPL). NHPL remains unaffected during water degumming due to their stable solubility in oil, not separating by precipitation or centrifugation [19-22]. HPL includes phosphatidylcholine, phosphatidylinositol, and lyso-phospholipids, while NHPL consists of phosphatidic acid and phosphatidylethanolamine, forming salts with divalent cations or existing in a dissociated form [22,23]. Modern degumming processes aim to target NHPL, converting them into HPL and effectively removing them. A simplified degumming scheme for vegetable oils involves the decomposition of phospho-metal complexes with the addition of acid, hydration of phospholipids with water, and partial neutralization with alkali [24].Depending on the vegetable oil type, refining process, and method, the tocopherol content in vegetable oil can decrease by up to 90%. Tocopherol losses are more significant in physical refining compared to chemical refining [25,26]. This is primarily due to the deodorization process occurring at temperatures exceeding 240°C, leading to substantial tocopherol losses. Thermal decomposition of tocopherols initiates at temperatures above 260°C [27]. In other refining stages, such as degumming and bleaching, tocopherol loss is relatively insignificant when compared to the deodorization process.The phosphorus content in refined oils typically falls below 10 mg/kg in terms of phosphorus, irrespective of the refining method. In physical refining, most phospholipids are removed through various methods such as TOP degumming, resulting in phosphorus content in oils below 10 mg/kg. In chemical refining, the primary removal of phospholipids, particularly water-hydratable phospholipids, occurs during water degumming, while another portion of phospholipids in vegetable oils is removed through caustic soda neutralization.Currently, in Central Asian countries, chemical refining of vegetable oils coexists with physical refining. This is mainly due to the prevalent use of cottonseed oil, which is primarily refined through chemical refining due to the presence of gossypol in crude oil. Sunflower oils are also used, and they may undergo either chemical or physical refining.
2. Materials and Methods
- Sunflower oil samples were collected from local oil factory Kattakurgan Oil Plant in Uzbekistan. The oilseeds were harvested in Kazahstan during april 2023. The refining process involves degumming, neutralizing, bleaching and physical refining process. Chemical refining processFor the water degumming process, crude sunflower oils were heated to 80°C, and a 4% by volume water was added to the mixture. This combination was then stirred for 30 minutes in a semi-continuous mixer with a flow rate of approximately 4 tons/h. Subsequently, the mixture was allowed to settle for 1 hour and then subjected to centrifugation using a Separator (QIE, China) at speed 4000 r.p.m. The water-degummed oil was then directed to a semi-continuous neutralizer. In the neutralization step, the degummed sunflower oils were again heated to 80°C. A 15% by weight sodium hydroxide (NaOH) solution, equivalent to 4% of the oil volume, was added to the mixture in the semi-continuous mixer at a flow rate of approximately 4 tons/h. After this addition, the mixture was left to settle for 1 hour and then underwent centrifugation using a Separator (QIE, China) at 4000 r.p.m. Following neutralization, the degummed oil was heated to 80°C and subjected to bleaching. This process involved the use of 0.5% w/w bleaching clay (Engelhard, Germany), The bleaching process was carried out with mechanical stirring under a vacuum of 11 x 103 Pa for 40 minutes. After bleaching, the oil was deodorized using a semi-continuous column (Muez Heist, India) at a rate of approximately 1 ton/h. The deodorization process took place at a temperature of 210°C, a vacuum of 470 Pa, and involved the direct injection of 35 kg/h of steam for 120 minutes (30 minutes).Physical refining processFor the physical refining process, crude sunflower oil was degummed using the TOP degumming method. In this process, the oil was treated with 0.3% w/w phosphoric acid (75% w/w), and the H3PO4 was neutralized with 0.15% w/w sodium hydroxide (40% w/w).Subsequently, the oil was heated to 80°C and bleached using 0.5% w/w bleaching clay (Engelhard, Germany) with mechanical stirring under a vacuum of 11 x 103 Pa for 40 minutes. The bleached oil was then physically refined under the same conditions as the chemical refining process, except for the temperature and steam injection parameters. In the physical refining process, the temperature was maintained at 240°C, and the direct steam injection rate was 45 kg/h.Determination phosphorus, tocopherols amount and acid value of vegetable oilsThe amount of phospholipids was determined as the total phosphorus on a vegetable oil according to AOCS Official Method Ca 12-55 [28]. The phospholipid content was determined under Thin-Layer Chromatography according to AOCS Ja-86 [29]. Acid value was determined according AOCS. Official method Cd 3d-63.: Acid value. 1993. Tocopherols were determined according AOCS. Official method Ce 8-89. Determination of tocopherols and tocotrienols in vegetable oil and fats by HPLC, 1993 [30].
3. Results and Discussion
- During the chemical refining process, the first step involved water degumming of sunflower oils. Water degumming of vegetable oils is widely used to remove phospholipids and was patented by Bollman in 1923 [31]. Crude extracted oil contained 251.1 mg/kg of phosphorus, which is more than two and a half times higher than that in the pressed oil, which contained 91.1 mg/kg (Table 1). As A. J. Dijkstra mentioned [22], water-non-hydratable phospholipids contain phosphatidic acid and phosphatidylethanolamine. Figure 1 shows the phospholipid content in extraction and press oils. Crude extracted sunflower oil contains 0.8% phosphatidic acid and 33.7% phosphatidylethanolamine, while crude pressed oil contains 0.1% phosphatidic acid and 41.7% phosphatidylethanolamine. After water degumming, the phosphorus content in extracted sunflower oil remained at 84.1 mg/kg, which is 33.4%, and the remaining phospholipids were removed. Also, the water-degummed pressed sunflower oil contains 38.1 mg/kg, which corresponds to a percentage content of 41.8% of non-hydratable phospholipids in the oil. Despite the fact that water degumming reduced the phosphorus content in the oil by more than 60%, the remaining amount of phosphorus can negatively affect the subsequent refining processes. In addition to phosphorus, crude sunflower oil contains a relatively large amount of tocopherols. Crude extracted oil contained 479.7 mg/kg of total tocopherols, and pressed oil contained 380.5 mg/kg (Table 2). Water degumming removed some tocopherols along with phospholipids and other impurities.
|
|
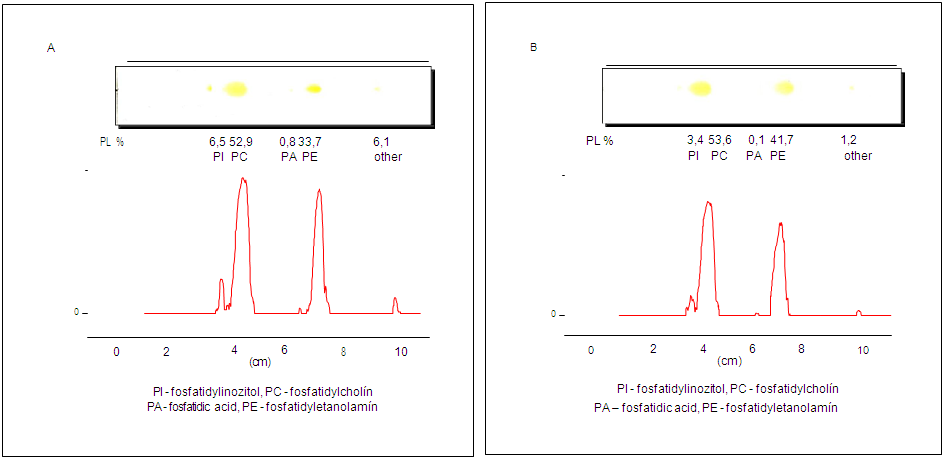 | Figure 1. Phospholipids content in crude extracted (A) and pressed (B) sunflower oils |
|
4. Conclusions
- The goal of this paper is analysing the effect of chemical and physical refining processes of sunflower oils. Chemical refining is a gentler process with respect to tocopherols. Physical reefing is more efficient in removing phosphorous but is associated with significant losses of tocopherols, which diminish the product nutritional value. Physical refining of sunflower oils involves only three stages: TOP degumming, bleaching and deodorization, while chemical refining involves four stages. Additionally, chemical reefing includes neutral oil saponification, leading to comparable losses compared to physical refining.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML