-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Polymer Science
p-ISSN: 2163-1344 e-ISSN: 2163-1352
2023; 12(2): 32-36
doi:10.5923/j.ajps.20231202.02
Received: Oct. 11, 2023; Accepted: Oct. 25, 2023; Published: Nov. 3, 2023

Sorption of Malachite Green by Polymer Composite Sorbents from Aqueous Solution
Umid Mirzakulov, Muzaffar Makhkamov, Islomjon Khudoyberdiyev
National University of Uzbekistan Named after Mirzo Ulugbek, Uzbekistan
Correspondence to: Islomjon Khudoyberdiyev, National University of Uzbekistan Named after Mirzo Ulugbek, Uzbekistan.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In this work, the sorption of malachite green (MG) from aqueous solutions by polymer composite gels (PCG) prepared on the basis of polyacrylic acid and bentonite clay (BG) was studied. It was found that the sorption capacity of PCG containing 50 weight % BG is almost three times greater than that of a gel not containing BG. Moreover, experiments have shown that the sorption capacity of PCG increases with increasing BG content in its composition. Adsorption isotherms were analyzed using the Langmuir and Freundlich models and, by comparing the standard deviations (R2), it was concluded that the sorption of MG compositions is better described by the Langmuir model.
Keywords: Polyacrylic acid, Polymer hydrogel, Bentonite clay, Composite polymer gel, Malachite green, Sorption
Cite this paper: Umid Mirzakulov, Muzaffar Makhkamov, Islomjon Khudoyberdiyev, Sorption of Malachite Green by Polymer Composite Sorbents from Aqueous Solution, American Journal of Polymer Science, Vol. 12 No. 2, 2023, pp. 32-36. doi: 10.5923/j.ajps.20231202.02.
Article Outline
1. Introduction
- As is known, polymer hydrogels (PH), containing charged groups are capable of absorbing and retaining huge amounts of water [1,2]. Such PHs have found application in various fields of science, national economy, medicine, pharmacology, biotechnology, etc. [3-5]. At the same time, research is also being conducted to improve the characteristics of PHs, which will lead to an even wider use of such materials. One of the ways to purposefully improve the physicochemical characteristics of PHs is to obtain polymer composite gels (PCGs) based on them. For this purpose, various components are introduced into the PH composition. Most often, substances such as silicates, carbon-containing particles, metal nanoparticles, etc. are used as components for the production of PCG. [6-9]. This makes it possible to purposefully improve the mechanical and physicochemical properties of PH. Previously, M.Makhkamov and other authors [10,11] obtained PCG based on polyacrylic acid and bentonite clay, and studied the structure, morphology and swelling in aqueous solutions. In this work, the sorption of the organic dye malachite green (MG) by PCG samples obtained on the basis of polyacrylic acid and BG was studied.
2. Experimental
2.1. Materials
- Acrylic acid (OAO Reaktiv, Russia) was purified by bidistillation. The crosslinking agent N,N'-methylene-bis-acrylamide (BDH Chemical Ltd, England), grade “pure grade” was used. Chemically pure reagents (Sigma-Aldrich; USA) were used to prepare aqueous solutions of MG. Alkaline bentonite clay (BG; Uzbekistan) PVB grade. Purification of BG from impurities was carried out using the water elutriation method proposed by S. Korolkova et al. [12] and Z. Gong et al. [13].
2.2. Synthesis of PG and PCG
- PG based on polyacrylic acid was synthesized according to the method given in the work of M.Makhkamov et al. [10]. PCG based on polyacrylic acid and BG was prepared according to the method given in the work of U.Mirzakulov et al. [11]. The resulting PH and PCG were purified by washing with distilled water for 20 hours in glass columns, after which they were dried at 318 K to constant weight.
2.3. Sorption
- The sorption of MG from aqueous solutions by sorbent samples was studied by the spectrophotometric method on a UV 5100 spectrophotometer (Metash, China). To do this, samples of sorbents of the same mass were placed in aqueous solutions of the corresponding salts and at certain intervals the change in the optical density of the solution was measured at a wavelength of 620 nm. The concentration of MG in the solution was determined based on calibration curves depending on the concentration of the MG solution on its optical density. The sorption value (qe, mmol/g) was calculated using the formula:
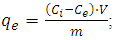 where: Сi and Сe – MG concentrations in solution before and after sorption, respectively, mmol/l; V– volume of solution, l; m– mass of sorbent, g.
where: Сi and Сe – MG concentrations in solution before and after sorption, respectively, mmol/l; V– volume of solution, l; m– mass of sorbent, g.3. Results and Discussion
3.1. Structure of PCG
- As is known, the mineral montmorillonite, which forms the basis of BG, has a layered structure that swells in polar solvents. Therefore, when preparing PCG, aqueous suspensions of BG were prepared and acrylic acid and a cross-linking agent (N,N-methylene-bis-acrylamide) were added to this suspension. Dissolved monomers also penetrate into the interlayer space of montmorillonite along with water molecules. When an initiator is added, the monomers polymerize to form a spatial network, the cells of which contain layers of montmorillonite (Fig.1).
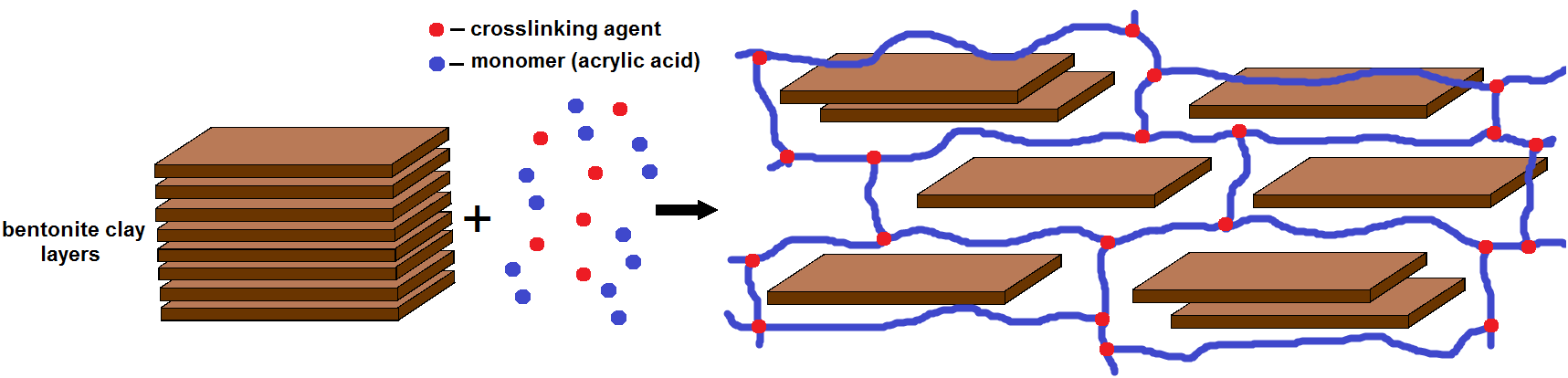 | Figure 1. PCG education scheme |
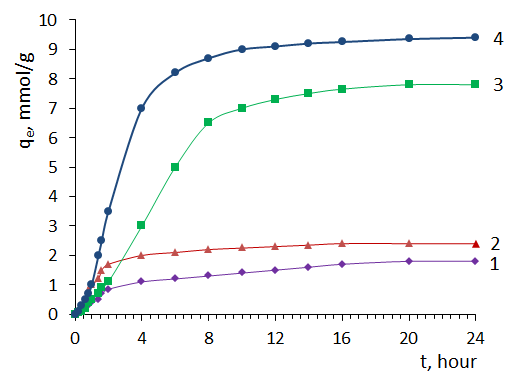
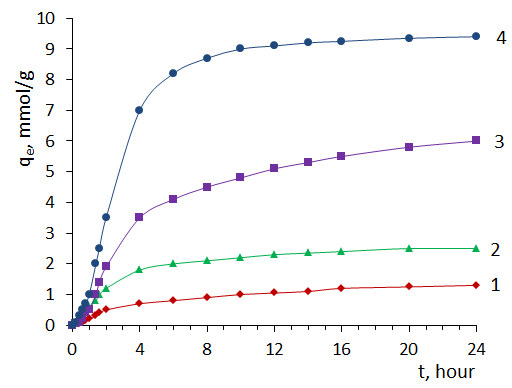
3.2. Sorption of MG by Sorbents from Solution
- Fig.2 shows the absorption spectrum of an aqueous solution of MG in the wavelength range 300-750 nm. As can be seen from the spectrum, the absorption maximum of the aqueous solution of MG is at a wavelength of 620 nm.
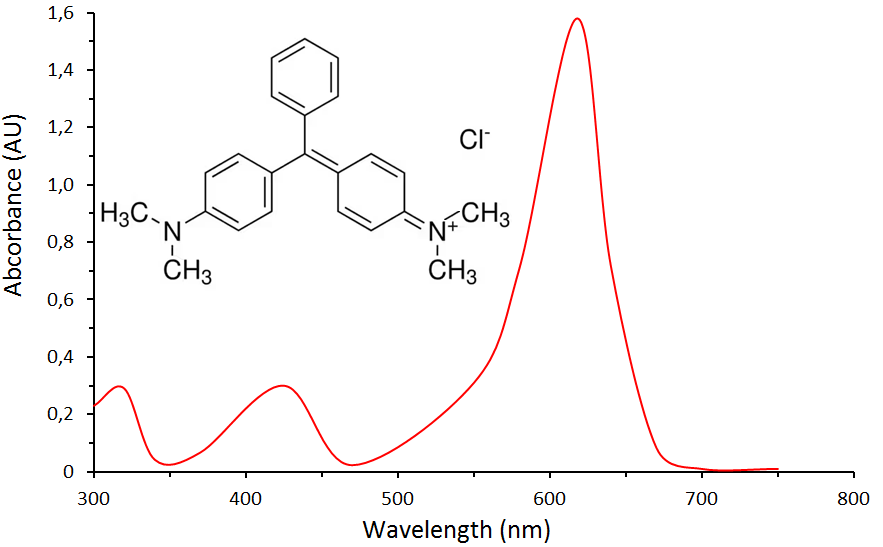 | Figure 2. Absorption spectrum of an aqueous solution of MG |
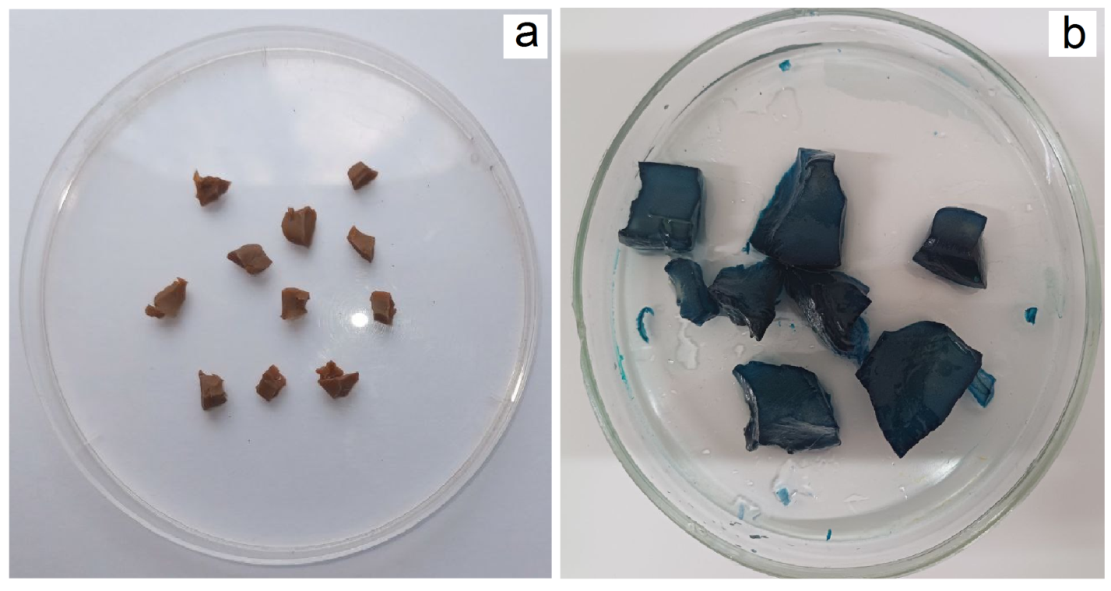 | Figure 5. Appearance of PCG (a) before and after sorption (b) MG from aqueous solution |
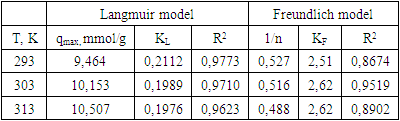
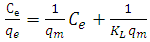 where: qe – adsorption value, mmol/g; qm – maximum adsorption value mmol/g; KL - Langmuir constant, l/mmol; Ce – equilibrium concentration, mmol/l.Linear correlations of Langmuir isotherms are shown in Fig.6. The main parameters calculated using this model are given in table 1.
where: qe – adsorption value, mmol/g; qm – maximum adsorption value mmol/g; KL - Langmuir constant, l/mmol; Ce – equilibrium concentration, mmol/l.Linear correlations of Langmuir isotherms are shown in Fig.6. The main parameters calculated using this model are given in table 1.
|
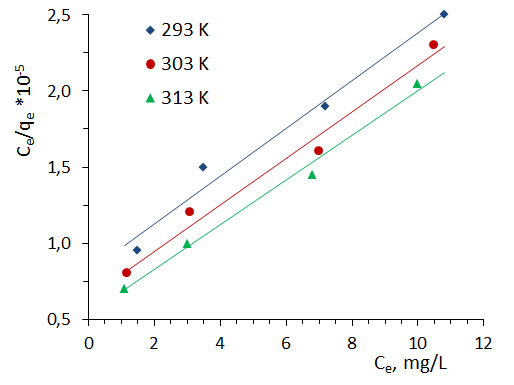 | Figure 6. Adsorption isotherms in coordinates of the linear form of the Langmuir equation. T=303K |
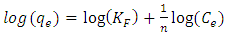 where: КF - the equilibrium constant of the Freundlich equation related to the adsorption capacity; 1/n – a parameter indicating the intensity of the adsorbent-adsorbate interaction.To describe the applicability of the model, the parameter 1/n is used, which is an indicator of the nature of adsorption: at 1/n=1, the distribution of adsorbed particles between two phases does not depend on concentration; at 1/n=0 adsorption is irreversible; at 1/n<1 adsorption is favorable, and at 1/n>1 adsorption is unfavorable. Linear correlations of Freundlich isotherms are shown in Fig.7. The main parameters calculated using this model are given in table 1.
where: КF - the equilibrium constant of the Freundlich equation related to the adsorption capacity; 1/n – a parameter indicating the intensity of the adsorbent-adsorbate interaction.To describe the applicability of the model, the parameter 1/n is used, which is an indicator of the nature of adsorption: at 1/n=1, the distribution of adsorbed particles between two phases does not depend on concentration; at 1/n=0 adsorption is irreversible; at 1/n<1 adsorption is favorable, and at 1/n>1 adsorption is unfavorable. Linear correlations of Freundlich isotherms are shown in Fig.7. The main parameters calculated using this model are given in table 1.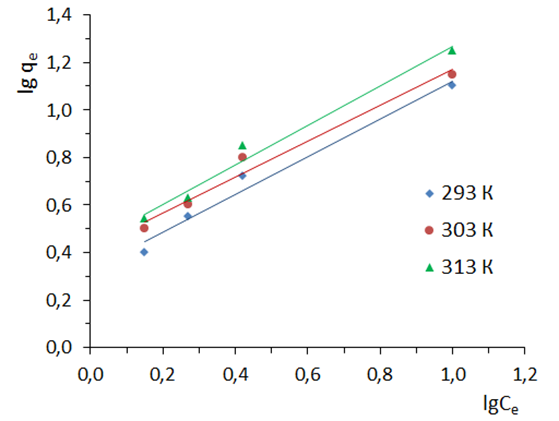 | Figure 7. Adsorption isotherms in coordinates of the linear form of the Freundlich equation. T=303K |
4. Conclusions
- Thus, the work studied the sorption of MG and its aqueous solutions by various sorbents, for which we used BG, PG based on polyacrylic acid, and PCG obtained by introducing BG into the composition of PH based on polyacrylic acid. Experiments have shown that the sorption capacity of PCG is much higher compared to BG and PH. At the same time, it was also revealed that an increase in the BG content in the PCG composition from 20 to 50 weight % leads to an increase in the sorption of MG from the solution. Sorption isotherms of MG compositions were analyzed using the Langmuir and Freundlich models. It was found that the Langmuir model is more suitable for describing the sorption process.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML