-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Polymer Science
p-ISSN: 2163-1344 e-ISSN: 2163-1352
2023; 12(2): 23-31
doi:10.5923/j.ajps.20231202.01
Received: Jun. 19, 2023; Accepted: Jul. 16, 2023; Published: Jul. 24, 2023

The Influence of Hydrogen Bonds on the Kinetics of Polymerization of Anilines and the Properties of the as-Produced Polyanilines
Matthew Tsonetokoy, Melissa Donley, Dell Fletcher, Chichi Nwatha, Maite Miller, Jake Rohrer, Louis Susanto, Bled N’Guessan, Roch Chan-Yu-King
University of Science and Arts of Oklahoma, Chickasha, OK, USA
Correspondence to: Bled N’Guessan, Roch Chan-Yu-King, University of Science and Arts of Oklahoma, Chickasha, OK, USA.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The influence of hydrogen bonds, mediated either by hexafluorosilic acid (H2SiF6) or potassium fluoride, dihydrate (KF.2H2O), in the oxidative polymerization of anilines is investigated. The reaction is conducted either under stirred or unstirred condition, in a glass or plastic vessel. The as-produced conducting polyanilines are analyzed by FT-IR, UV-Vis, cyclic voltammetry, SEM and elemental analyses. The electrical conductivity of the doped polyanilines was found to lie within the usual range of those synthesized through conventional methods. The kinetics of the polymerization are monitored via an open circuit profiling technique. The results indicate that fluoride ions can drastically slow down the reaction kinetics. And that both fluoride ions and H2SiF6 can impart nanomorphology in the bulk polyanilines whose fibers have an average diameter of ca. 100 nm.
Keywords: Kinetics of aniline polymerization, Hydrogen bonds in polymerization of anilines, Influence of fluorides, Hexafluorosilicate ions or hexafluorosilic acid on polymerization of anilines, Hexafluorosilic acid as dopant of polyanilines, Nanofibers of polyanilines
Cite this paper: Matthew Tsonetokoy, Melissa Donley, Dell Fletcher, Chichi Nwatha, Maite Miller, Jake Rohrer, Louis Susanto, Bled N’Guessan, Roch Chan-Yu-King, The Influence of Hydrogen Bonds on the Kinetics of Polymerization of Anilines and the Properties of the as-Produced Polyanilines, American Journal of Polymer Science, Vol. 12 No. 2, 2023, pp. 23-31. doi: 10.5923/j.ajps.20231202.01.
Article Outline
1. Introduction
- Polyanilines (PANI) is one of the most studied intrinsically conducting polymers because of its simple synthetic protocol, facile processability, good stability and relatively high electrical conductivity. The three commonly cited oxidation states of PANI are summarized in Fig. 1 where x, y and 1-y indicate the degree of polymerization, the reduced and oxidized repeat units, respectively. The completely reduced state of PANI is leucoemeraldine base (LEB) with 1-y = 0. The fully oxidized state (1-y = 1) is pernigraniline base (PB) and that of the half-oxidized state is emeraldine base (EB) with 1-y = 0.5 [1].
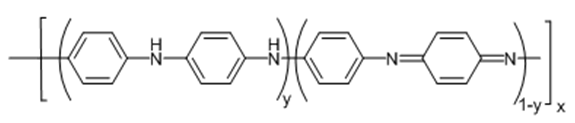 | Figure 1. Structure of polyanilines (PANI) |
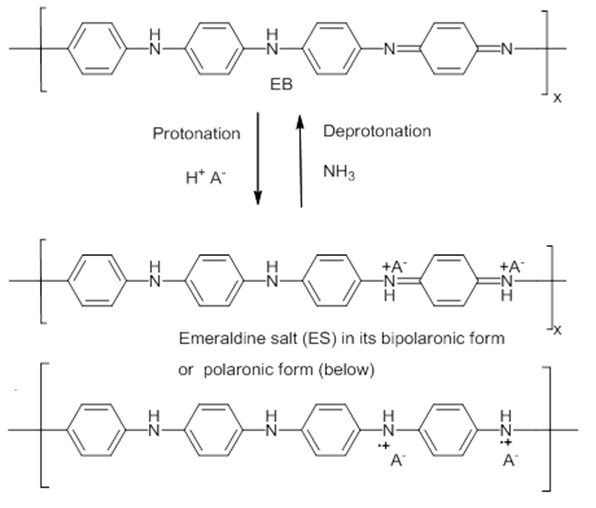 | Figure 2. Processes of doping of EB with an acid and dedoping of ES with a base. In the ES structure, the A- is the dopant anion |
2. Experimental
2.1. Materials
- All chemicals were purchased from Sigma-Aldrich Co. and were used as received except for aniline which was freshly distilled under reduced pressure prior to polymerization.
2.2. Instrumentation
- FTIR and UV-Vis spectra are recorded with the use of a Nicolet iS10 (Thermoscientific Corporation) and UV-2550 spectrometers (Shimadzu Co.), respectively. Electrical conductivity of pelletized ES is measured with a four-probe device connected to a Lakeshore 120CS DC microcurrent source which is coupled to an auto-ranging multimeter 175A (Keithley/Tektronix Co.). Morphological characteristics of PANI are obtained via SEM (Zeiss Neon EsB, operating at 5 KV). The electron microscope is equipped with an energy dispersive X-ray spectrometer (EDS) operating at 10-15 KV. Prior to SEM analyses, the ES were briefly sonicated in DI water. A suspension was retrieved via pipet and placed on a silicon wafer. The sample was then air-dried. The kinetics of polymerization is monitored via an open circuit potential (VOC) profiling technique [7] using a concentrated agar/KCl(aq) electrolyte solution as the salt bridge (Pt electrode and SCE as reference). The electrolyte solution (contained in a separatory funnel) is connected to the polymerizing solution via a plastic tubing with one end attached to an Eppendorf (plastic) pipette whose tip is immersed into the reaction bath. Quantitative elemental analyses are from Micro-Analysis, Inc. Cyclic voltammetry (CV) is conducted with a WaveNow 50-EDU potentiostat from Pine Instrument Co. (using pre-printed carbon electrodes with silver/silver-chloride as reference electrode). For CV experiments, aqueous solutions of the ES are briefly sonicated, then drop casted onto the working electrode and subsequently air dried. The electrode was then immersed in a 1.0 M aq.HCl (electrolyte) bath for voltammetry measurements (scan rate of 20 mv/sec).
2.3. Preparation of PANI in the Presence of Fluoride Ions in HCl (aq), a Mixed Halide Medium
- Care is exercised when conducting the polymerization as etching agents are formed in-situ. Gloves and safety goggles are worn. All reactions are carried out in a well-ventilated fume hood. Typical experimental protocols are given below.
2.3.1. Polymerization under Stirred Condition
- A mixture of 0.34 ml (3.77 mmol) aniline and 6.00g (64.00 mmol) of KF.2H2O in 75 ml of 1.0M HCl (aq) was stirred in a 250-ml (glass or plastic) beaker for 15 min. Into this stirred solution was added, dropwise, a solution of 0.95g (4.16 mmol) APS in 70 mL of 1.0 M HCl (aq). The resulting mixture was further stirred for 4 h after which time it was suction filtered on a Buchner funnel. The deep green-, doped ES was sequentially washed with copious amounts of DI water and acetone until a colourless filtrate was obtained. The collected polymer was high vacuum dried at 50°C for 4 h and stored in a dessicator.
2.3.2. Polymerization under Unstirred Condition
- The above reaction was repeated in a glass or plastic beaker. The anilinium in HCl(aq) containing 0.5g KF.2H2O (5.31 mmol) was stirred briefly for a few minutes and the magnetic stir bar was then removed. This was followed by a dropwise addition of the APS (aq) solution from a separatory funnel. The resulting mixture was left undisturbed for 48h after which time it was suction filtered and worked up as described in 2.3 a above.
2.3.3. Polymerization of Anilines in aq. Hexafluorosilic Acid (H2SiF6)
- Into a 150-ml plastic beaker was placed 75 ml (ca. 160 mmol) H2SiF6 (20-30% in water), followed by a slow dropwise addition of 0.34 ml (3.77 mmol) aniline. Some white solid particulates precipitated out. They were pulverized with a spatula and the suspension is stirred until all solids dissolved. Then a solution of 0.95g (4.16 mmol) APS (aq) in 60 ml water was added dropwise in ca. 30 min. The resulting mixture was stirred for 4 h, suction filtered and worked up as described in 2.3a above.
2.3.4. Kinetic Study of Polymerization via Open Circuit Potential (VOC)
- The experiment described in section 2.3.a above was repeated with 6.00g (64.00 mmol) KF.2H2O either in a plastic or glass vessel under stirring (500 rpm). A conventional polymerization (without KF) is also conducted in HCl (aq). In a separate polymerization, the experiment described in 2.3.c was also repeated in a plastic beaker. In all experiments, the solution of APS was added dropwise into the acidic anilinium solution in 5 min. The progress of the polymerization is monitored by recording the VOC change of the solution vs. time as soon as the first drop of the APS solution was added. The stirring was stopped when the voltage became constant.
3. Results and Discussion
3.1. Spectral Characterization
- We first carried out a series of aniline polymerization in HCl (aq) in a glass beaker containing one of the following amounts of KF.2H2O: 0.5, 2.0, 4.0 and 6.0 g. All isolated polymers exhibit the typical deep green colour which is a characteristic of PANI in its doped ES state. They are invariably mixed with some impure white/greyish particulates, referred here after as the “by-product”. A representative IR spectrum of the impure ES is displayed in Fig. 3a. It shows vibrations with wavenumber (cm-1) of 482.05, 741.40, 1129.43 (C-H in plane stretch), 1296.11 (C-N stretch, benzenoid), 1485.46 (C=C stretch benzenoid) and 1563.65 (C=C stretch, quinoid). The two prominent bands at 482.05 and 741.40 cm-1 are foreign to a conventionally synthesized ES, prepared in HCl (aq)) whose typical spectrum (Fig. 3b) exhibits absorptions with wavenumber (cm-1) of 504.69, 617.31, 819.47, 1138.10 (C-H in plane stretch), 1299.50 (C-N stretch, benzenoid), 1491.71 (C=C stretch benzenoid), 1577.41 (C=C stretch, quinoid) and 3448.82 (N-H stretch).
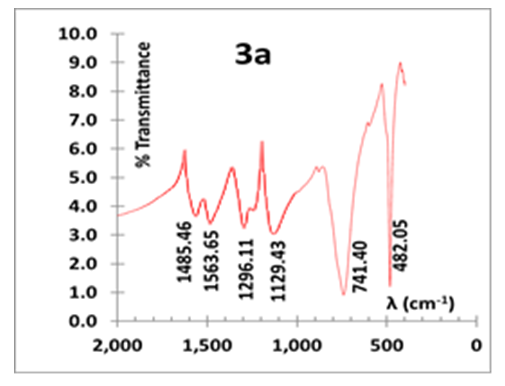 | Figure 3a. IR of impure ES (prepared in 6g KF/HCl medium) |
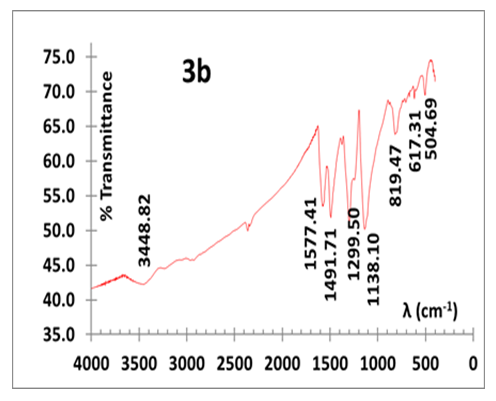 | Figure 3b. IR of ES prepared in HCl medium (conventional method) |
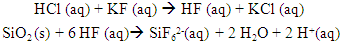 | (1) |
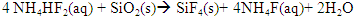 | (2) |
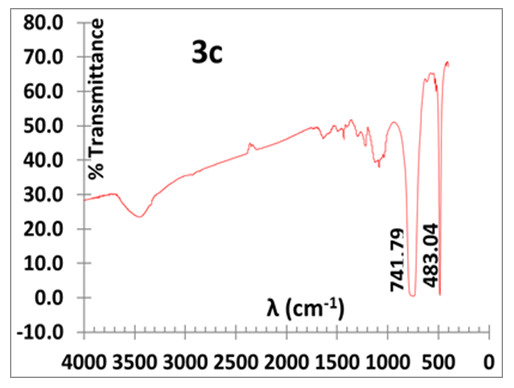 | Figure 3c. IR of isolated by-product |
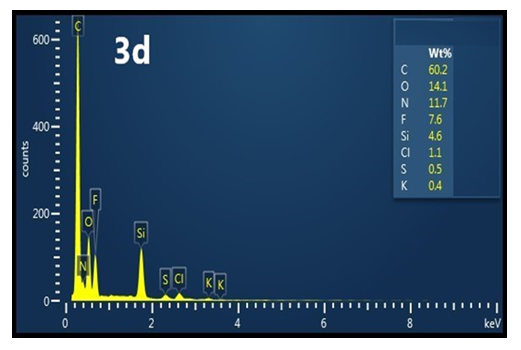 | Figure 3d. EDS of an impure ES |
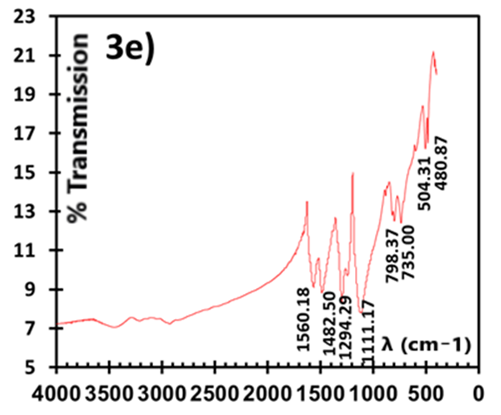 | Figure 3e. IR spectra of ES (prepared in H2SiF6) |
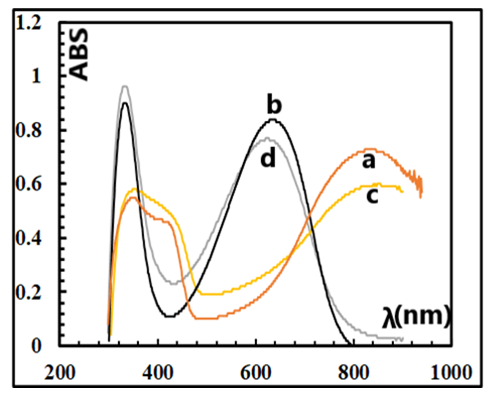 | Figure 4. UV spectra of ES (4a) and EB (4b) prepared in H2SiF6 and those of ES (4c) and EB (4d) prepared in HCl/KF.2H2O |
3.2. Kinetic Study
- The kinetics of polymerization are monitored through the change of the reaction-medium open circuit potential (VOC) vs time. The results are displayed in Fig. 5 along with the characteristics of the corresponding plots (Table 1).
|
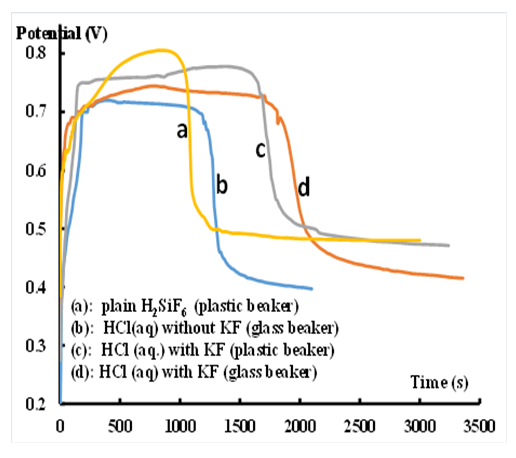 | Figure 5. Potential vs. time profiles of aniline polymerizations |
3.3. Cyclic Voltammetry
- Representative cyclic voltammograms of ES samples prepared in plastic beakers, doped either with plain HCl, HCl/6g KF.2H2O or plain H2SiF6 (aq), are displayed in Fig 6 (a), (b) and (c), respectively. The plots resemble those of the ES reported by Jugovic et al [31] who polymerized anilines on graphite electrodes. They exhibit the two sets of anodic and cathodic current peaks, typical of conducting PANI. The first set (ox1 and red1) is associated with the conversion of the fully reduced leucoemeraldine to the partially oxidized emeraldine and the second set (ox2 and red 2) corresponds to the conversion of the emeraldine state to the fully oxidized pernigraniline state [32]. In the case of ES doped with H2SiF6, its voltammogram shows one additional redox couple (ox 3 and red 3). This redox couple could be due to a) the degradation of by-products of PANI such as benzoquinone or hydroquinone [33] or b) the presence of either PANI containing insoluble quinonic groups or polymers from ortho-coupling of phenazine containing units [34].
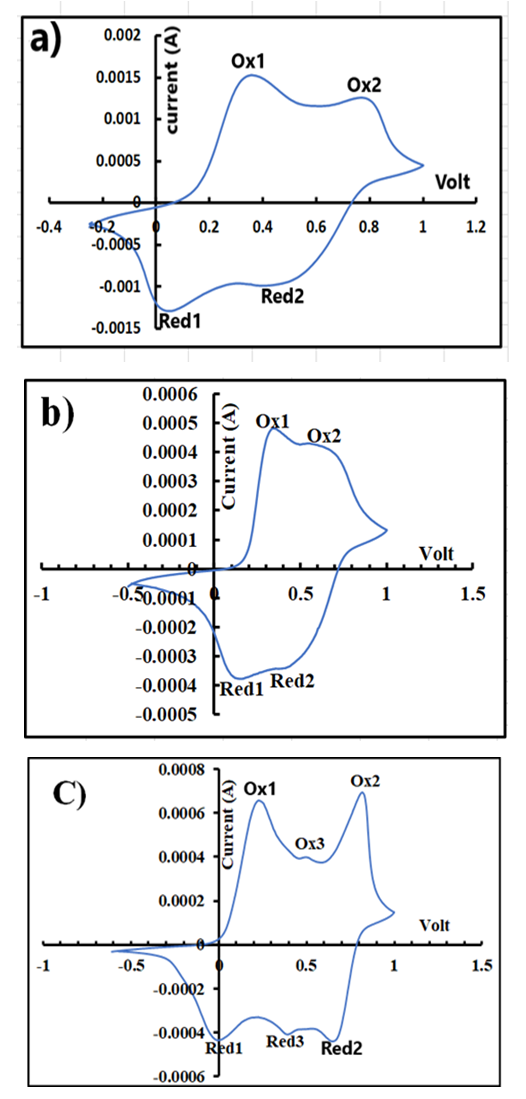 | Figure 6. Voltammograms of an ES sample prepared in a) HCl (aq), b) HCl(aq)/KF.2H2O, c) H2SiF6 (aq) |
3.4. Electrical Conductivity
- The electrical conductivity of representative ES samples was measured via the four-probe technique. For samples prepared in HCl (aq) containing KF.2H2O (glass beaker). The results are as follows: 3.00 S/cm, (0.5g), 0.61S/cm, (2g); 0.44S/cm (4g) and 0.60S/cm (6g). Those of the ES synthesized in plain HCl (glass vessel) and plain H2SiF6 (plastic vessel) are 1.10S/cm and 4.80 S/cm, respectively. Overall, the conductivity values are comparable to and fall within the usual reported range (10-2 to 102 S/cm) of most ES prepared under the conventional method [20].
3.5. Morphological Analyses of PANI via SEM and Elemental Analyses via EDS
- We have explored various experimental conditions that might influence the morphology of the ES. Representative SEM pictures of the synthesised polymers prepared in HCl/KF are displayed in Fig 7. With 0.5 g of KF.2H2O, the ES, obtained under unstirred conditions for 48 h, are composed mostly of nanofibers whose average diameters are less than 100 nm (Fig 7a and 7b). The nature of the vessel (glass vs plastic) does not seem to significantly affect the morphology of the polymers. This supports our previous findings that the by-products formed from glass etching processes must have precipitated quickly at the initial stage of the polymerization and they do not influence the polymer growth/final morphology. With 2g of KF.2H2O (stirred conditions, 3.5 h), the as-formed ES is essentially nanofibrous in nature (Fig 7c) although slightly shorter than those described earlier. With 4g of KF.2H2O, the ES gradually becomes less homogeneous as larger/globular features are formed along with the nanofibers (Fig 7d). The morphology became much less regular when the reaction was conducted in the presence of 6g of KF.2H2O (Fig 6e). This observation suggests that the first formed ES had served as templates for secondary polymeric growth as evidenced by the presence smaller grains deposited on the surface of the fibers. An SEM picture of an ES sample doped with H2SiF6 (plastic vessel) is also presented in Fig 7f. The fibers are by far more homogeneous in their average lengths and diameters (<80nm in average diameters) than those of its counter parts presented earlier. This is evidenced by the presence of thin and elongated nanofibers. In summary, we believe the afore-mentioned numerous H-bonds taking place, during polymerization, between F- or H2SiF6 and the monomers or oligo- or polyanilines, are instrumental in producing chain like structures which then direct the elongation of the polymers to eventually yield the observed nanomorphology. Without these strong H-bonds, only granular PANI is formed (Fig 7 g) as has frequently been observed under the conventional, stirred polymerization condition (glass beaker) [35], [36]. While we have not investigated the influence of temperature on morphology of ES, it has been reported that the polymerization of anilines in plain HF (aq) at 5°C produced an ES with porous film like morphology [37].
 | Figure 7(a). with 0.5g KF in HCl (unstirred, glass beaker 48 h reaction) |
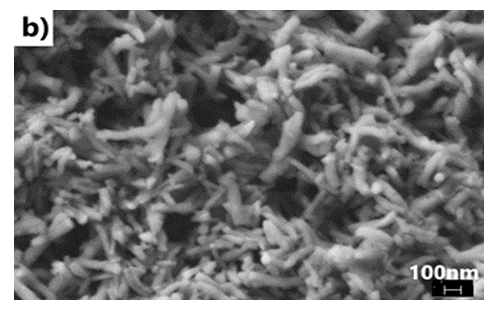 | Figure 7(b). with 0.5g KF in HCl (unstirred, plastic beaker, 48 h reaction) |
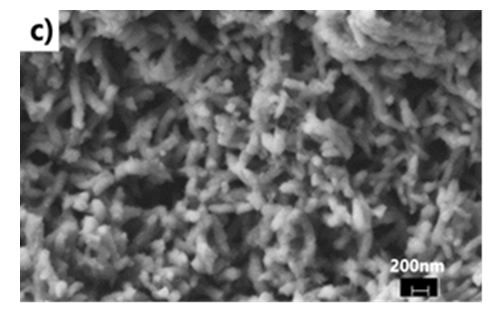 | Figure 7(c). with 2g KF in HCl (stirred, glass beaker, 4 h reaction) |
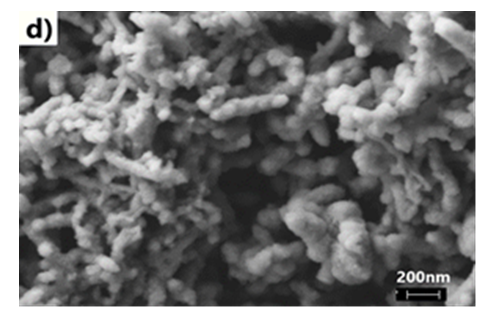 | Figure 7(d). with 4g KF in HCl (stirred, glass beaker, h reaction) |
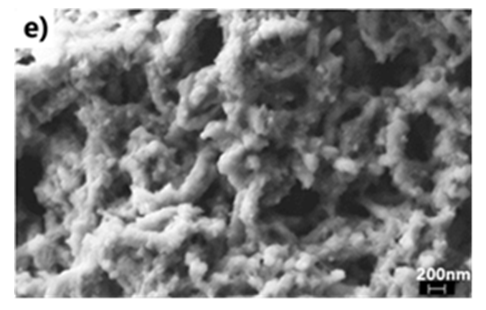 | Figure 7(e). 6g KF in HCl (stirred, glass beaker, 4 h Reaction) |
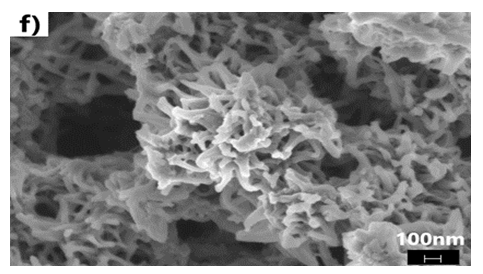 | Figure 7(f). In plain H2SiF6 (stirred, plastic beaker, 4 h reaction) |
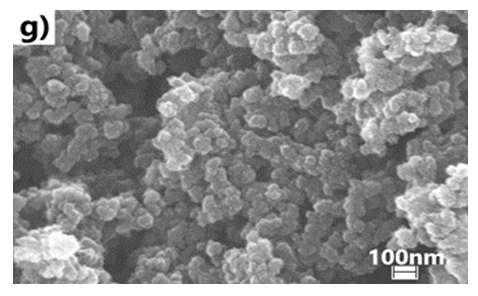 | Figure 7(g). In plain HCl (stirred, glass beaker, 4h reaction) |
4. Conclusions
- The influence of H-bondings from either fluoride ions or hexafluorosilicic acid on the kinetics of the polymerization anilines and the morphology of the as-formed PANI has been assessed. It is found that the morphology of PANI, prepared under the conventional condition (in plain HCl), is granular while those prepared in plain hexafluorosilicic acid or in a mixed halide medium (F- and Cl- from HF and HCl) are essentially nanofibrous at low concentration of fluorides or in plain hexafluorosilicic acid. Despite the etching of glass beaker when HF (aq) is present, it is found that the morphology of the PANI produced is independent of the vessel used (glass or plastic beaker). However, etching and H-bondings between reagents and/or glass walls affect the kinetics of polymerization and the oxidation potential of the reacting species as evidenced by open circuit potential vs time profiling of the polymerization. The results indicate that the polymerization is a) fastest in plain hexafluorosilicic acid (plastic beaker) and b) slowest in HCl(aq)/HF (aq) medium (glass beaker). The electrical conductivity (0.10 S/cm < σ <10 S/cm) of all prepared PANI lies within the usual range of that of a conventionally prepared sample.
ACKNOWLEDGEMENTS
- The authors thank the Foundation of the University of Science and Arts of Oklahoma for its financial contribution through the Gladys Emerson Research Fund.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML