Pulatova Nilufar Ubaydullayeva1, Maksumova Oytura Sitdikovna2
1Doctor of Philosophy in Chemical Sciences (PhD), Tashkent Institute of Chemical Technology, Tashkent, Republic of Uzbekistan
2Doctor of Chemical Sciences Professor, Tashkent Institute of Chemical Technology, Tashkent, Republic of Uzbekistan
Correspondence to: Pulatova Nilufar Ubaydullayeva, Doctor of Philosophy in Chemical Sciences (PhD), Tashkent Institute of Chemical Technology, Tashkent, Republic of Uzbekistan.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
The article presents the results of the copolymerization process of 1-chloro-3-piperidino-2-propylacrylate (XPPA) and acrylic acid (AA). The effect on the copolymerization process of such factors as the nature of the solvent and the ratio of the initial monomers has been studied. The optimal conditions for the synthesis of copolymers under conditions of radical polymerization in the temperature range of 60-80°C are found. The values of the relative activities of the monomers were calculated by the Fineman-Ross method, which are 𝑟1=0,88, 𝑟2=0,66. The acute toxicity and antimicrobial activity of these synthesized copolymers were studied and it was found that the test compounds according to the parameters of acute toxicity according to the classification of IHS 12.1.007-76 belong to the IV class of low-toxic substances and have an antimicrobial effect against St. Epidermidis, E. coli и Bac. Subtilis.
Keywords:
1-chloro-3-piperidine-2-propylacrylate, Acrylic acid, Polymer, Initiator, Kinetic parameters, Temperature, Antimicrobial activity
Cite this paper: Pulatova Nilufar Ubaydullayeva, Maksumova Oytura Sitdikovna, Properties and Antibacterial Properties of a Copolymer Based on 1-Chloro-3-Piperidine-2-Propylacrylate and Acrylic Acid, American Journal of Polymer Science, Vol. 12 No. 1, 2023, pp. 1-6. doi: 10.5923/j.ajps.20231201.01.
1. Introduction
Polymers have long gained a strong position in various fields of medicine and biology, and in the future, the possibilities of their use are unlimited. The development of methods for the synthesis and modification of polymers and copolymers has made it possible to approach the solution of the most important problems of practical medicine. The role of polymers in pharmacology is especially great in the creation of drugs with a controlled release of a biologically active component. [1,2]. Copolymerization reactions based on acrylic acid and acrylic acid esters in various solvent and non-solvent media are currently being actively studied. [3-5]. Copolymers based on acrylic acid (AA) and acrylic acid esters are widely used in various industries, including as thickeners, emulsifiers, and stabilizers of the physicochemical properties of the environment [6,7]. However, despite their great practical importance, monomers for the production of multifunctional copolymers are very rare, and the properties of these compounds have been little studied.The purpose of this work is to study the radical copolymerization of 1-chloro-3-piperidine-2-propylacrylate and acrylic acid and to study the toxicity and antibacterial activity of the synthesized copolymers.
2. Research Methods
IR spectra of the starting reagents and synthesized substances were obtained on a SHIMADZU IR-100 spectrometer. The FT-IR spectrometer of the synthesized polymers was obtained on a Cary 630 spectrometer (Agilent Technologies, company no. MY 14270025) brand. It measures the absorption spectra of substances in the range from 5000 to 400 cm-1. The thermoanalytical study of the synthesized samples was carried out on a Netzsch Simultaneous Analyzer STA 409 PG instrument (Germany) with a K-type thermocouple and aluminum crucibles (Low RG Silver). Mass spectrometry. Mass spectrum 6420 Triple Quad LC/MS (Agilentc Technologies, USA) was obtained by APCI ionization (atmospheric pressure chemical ionization). Thermal analysis. The thermal stability of the polymers of the synthesized monomers and hydrogels based on them, as well as combustible coatings, was analyzed by differential thermal and thermogravimetric methods on an instrument from the Japanese company SHIMADZU [8,9]. SHIMADZU (Simultaneous Heat Analysis) TGA as well as TGA-DTA, TGA-DSC analysis methods provide an easy-to-use, reliable and high performance thermal analysis platform. The derivatograph was studied at a speed of 10 deg/min, T-900, TG-200, DTA - 1/10, DTG - 1/10 galvanometric sensitivity, by automatically recording the derivatogram on photographic paper. A weighed portion of the studied pigments weighing 35–46 mg was placed in a crucible made of aluminum oxide and platinum, resistant to a temperature of 1650°C, without a lid 10 mm in diameter.
3. Results
The studies were carried out by the gravimetric method. This method separates the resulting copolymer by precipitation in the presence of various solvents [10]. The copolymerization reaction was carried out in an organic solvent medium at a temperature of 60–80°C in the presence of a radical initiator DAK. Dimethylformamide was used as a solvent. The copolymerization process was carried out in a special ground heat-resistant test tube. In clean test tubes, the required amount of the initiator (DAK) was dissolved in the solvent, after which 1-chloro-3-piperidine-2-propylacrylate and acrylic acid were added in the required proportions. The mixture was stirred until complete dissolution of the initiator, then gaseous nitrogen was passed through for 10 min, after which the glass tubes were carefully stoppered. After that, it was placed in a thermostat with a given temperature (60-80°C). The copolymerization reaction was carried out at a degree of conversion of 10–12%, which was determined upon reaching a thick mass [11]. After that, the ampoules were cooled to room temperature and precipitated into benzene. Copolymer samples were dried and weighed to constant weight on an analytical balance with an accuracy of ±0.0002. The obtained copolymers are white powder products, soluble in water, ethanol, dimethylformamide. The monomer 1-chloro-3-piperidine-2-propylacrylate was synthesized according to the procedure described in [12].The structure of the resulting poly-1-chloro-3-piperidine-2-propyl acrylate (CLPAA) was studied by IR spectral analysis (Fig. 1).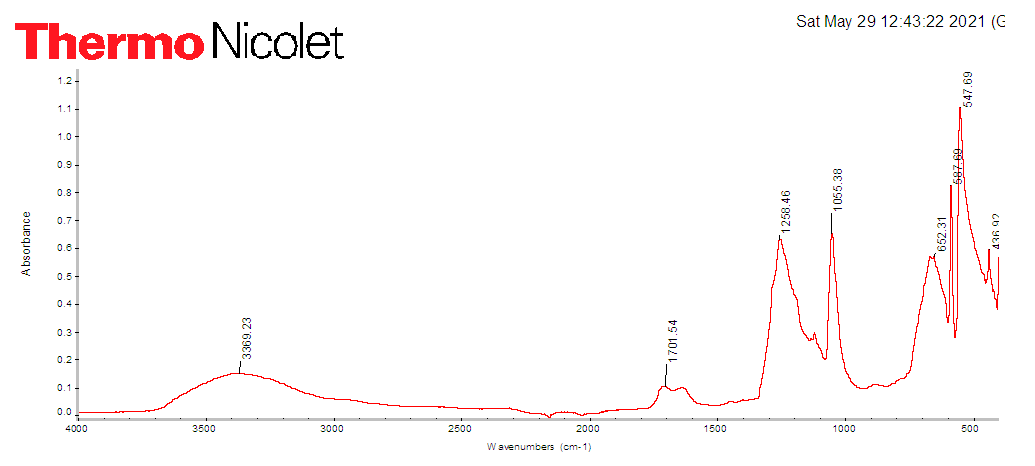 | Figure 1. IR spectrum of poly-1-chloro-3-piperidine-2-propyl acrylate |
The IR spectroscopy data confirm the preparation of a new CLPAA polymer. In the spectrum of polymer samples, absorption bands are found that are characteristic of fragments of both CLPAA in the regions of 1701 cm-1 (ester), at 1258 cm-1 stretching vibrations of the carbonyl group С=О, and bending symmetric vibrations of CH2 and CH3 at 1055 cm-1 [12]. The absence in the IR spectra of the copolymers of absorption bands characteristic of double C=C bonds indicates that the polymerization reaction proceeds via the vinyl groups of the initial monomers. The copolymerization reaction of CLPAA with AA can be represented by the following scheme: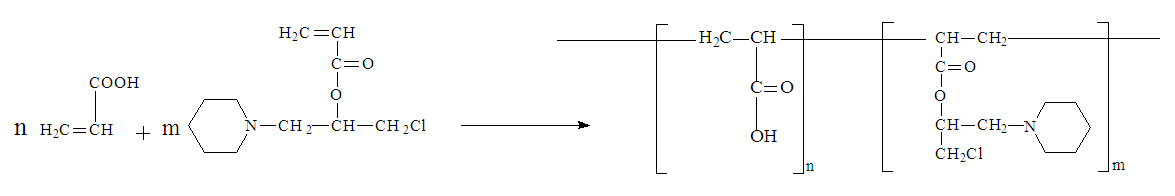 | Scheme |
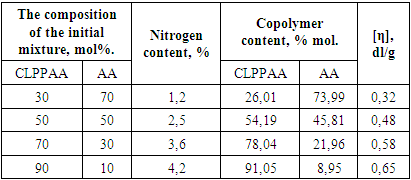
The effect of the ratio of initial monomers on the copolymerization reaction was studied (Table 1).Table 1. Copolymerization of CLPPAA and AA in DMF solvent medium (DAK =5·10-3 mol/l, 80°C, 2 hours)
 |
| |
|
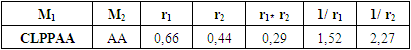
Based on the composition of the copolymers found in different ratios of the initial mixture, the values of the copolymerization constants were calculated CLPPAA (r1) и АA (r2) [13, p. 100]. They were determined on the basis of experimental data, by graphical solution of the Feineman-Ross differential equation (Table 2).Table 2. The value of copolymerization constants CLPPAA (М1) and AA (М2)
 |
| |
|
From the obtained values of the copolymerization constants, it can be seen that r1<1 and r2<1. According to the obtained data, CLPPAA is a more active monomer than AA in the reaction of radical copolymerization.The thermal stability of sopolymers was studied by differential thermal and thermogravimetric methods (Fig. 2).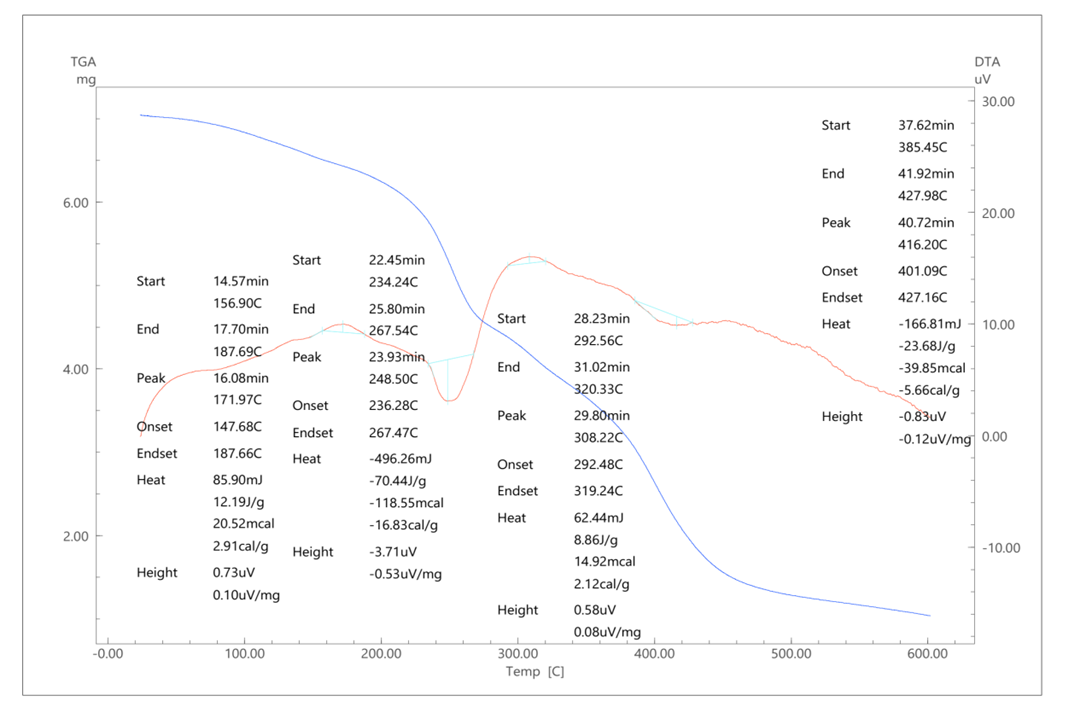 | Figure 2. Thermogram of CLPPAA and AA sopolymer at 75°C |
Differential modes of heating and spheroid samples were carried out in the atmosphere at temperatures up to 200 - 600°C. From here, the endothermic and exothermic points of the samples were confirmed [13-14]. As can be seen from Figure 2, the DTA curve of the sopolymer based on CLPPAA and AA is characterized by an endothermic maximum at 234°C and the presence of one glass transition region (267°C).The acute toxicity of the synthesized compounds of CLPPAA with AA at their ratio of 1:1 was studied on 30 white mice, weighing 19-21 g, of mixed sex. The copolymer compound was administered to mice once intragastrically at doses of 3000 mg/kg, 5000 mg/kg and 7000 mg/kg (0.1-0.5 ml) [14].Animals were monitored continuously for the first hour, then hourly during the first day of the experiment, and once a day for the next 13 days of the experiment. All experimental animals were kept under standard conditions, on a general diet with free access to water and food. [15]. After the completion of the experiment, the average lethal doses (LD50) were determined. LD50 of said copolymer was 450.0 (385.4÷514.6) mg/kg.The antimicrobial activity of a copolymer based on 1-chloro-3-piperidine-2-propylacrylate with acrylic acid was determined by the method of diffusion into agar on a dense nutrient medium by comparing the sizes of zones of growth inhibition of test microbes formed during testing of solutions of the test sample [16-18].Sterile Petri dishes of the same diameter with a flat flat bottom were used for analysis. 20 ml of a nutrient medium of a certain composition, infected with an 18-20 hour culture of test strains, was poured into cups installed on a horizontal table. (Staphylococcus epidermidis, Staphylococcus aureus, Bacillus subtilis, Escherichia coli). Appropriate nutrient media were used for research.Preparation of the inoculum: for the preparation of the inoculum, pure daily cultures of microorganisms grown on solid nutrient media were used. Several similar, clearly isolated colonies were selected. A small amount of material from the tops of the colonies was transferred with a loop into a test tube with a sterile 0.9% NaCl solution, bringing the density of the inoculum to exactly 0.5 according to the McFarland standard. The inoculum was used within 15 minutes of preparation.Analysis: 0.02% and 0.03% solution of the test sample of the copolymer based on 1-chloro-3-piperidine- 2-propylacrylate with acrylic acid was prepared for testing. Wells were made on the frozen surface of agar, in the center with a glass cylinder. The test samples were added to the wells in six Petri dishes.IncubationThe cups were placed in a thermostat at a temperature of (36 ± 1)°C for 18-24 hours. After incubation in a thermostat, the zones of inhibition of the growth of microorganisms formed by 1%, 2%, 3% solutions of a copolymer based on 1-chloro-3-piperidine-2-propylacrylate with acrylic acid were measured with a microbiological ruler with an accuracy of 1 mm. The microbiological activity of the test agent was assessed by the size of the zones. After incubation in a thermostat, the zones of inhibition of the growth of microorganisms formed by the solutions of the test agents were measured with a microbiological ruler with an accuracy of 1 mm. The microbiological activity of the test agents was assessed by the size of the zones (Table 3,4,5).Table 3. Microbial growth inhibition zones exposed to a 1% solution of a copolymer based on 1-chloro-3-piperidine-2-propylacrylate with acrylic acid
 |
| |
|
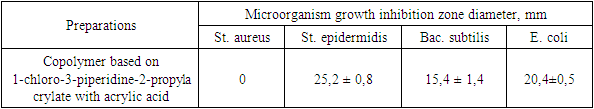
Table 4. Zones of inhibition of growth of microorganisms under the influence of a 2% solution of a copolymer based on 1-chloro-3-piperidine-2-propylacrylate with acrylic acid
 |
| |
|
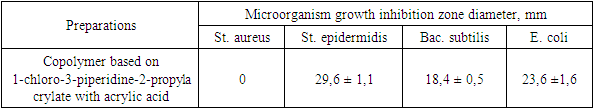
Table 5. Zones of inhibition of growth of microorganisms under the influence of a 3% solution of a copolymer based on 1-chloro-3-piperidine-2-propylacrylate with acrylic acid
 |
| |
|
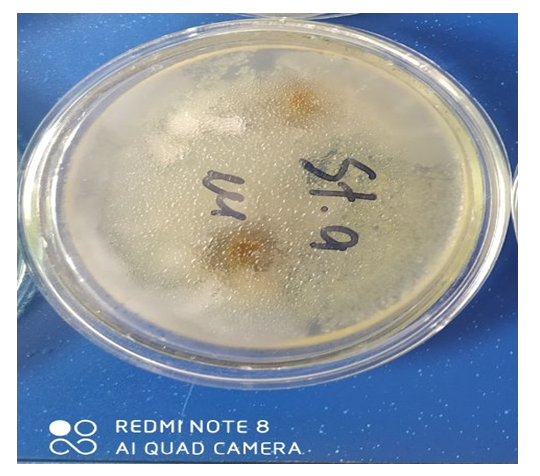
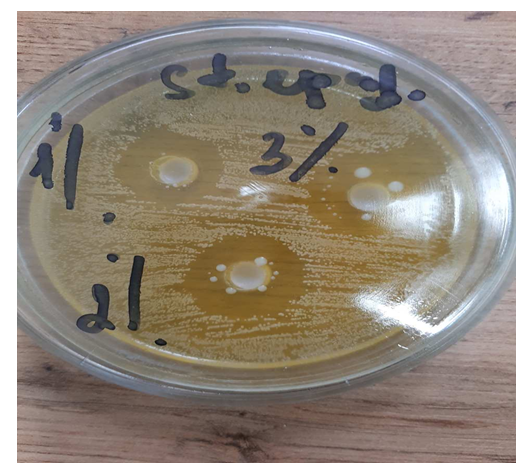
The data obtained show that a 3% solution of a copolymer compound based on 1-chloro-3-piperidine-2-propylacrylate with acrylic acid has a more pronounced antimicrobial effect than 1% and 2% copolymer solutions. | Figure 3. Zones of inhibition Bac. subtilis |
 | Figure 4. Zones of inhibition St. аureus |
 | Figure 5. Zones of inhibition St. Epidermidis |
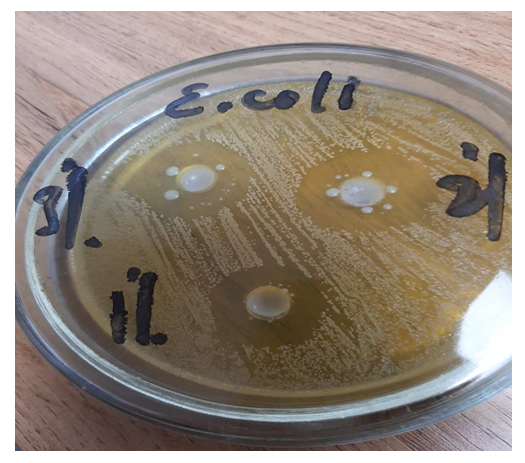 | Figure 6. Zones of inhibition E. coli |
4. Conclusions
1. The effect of the nature of the solvent and the ratio of the starting monomers on the process of radical copolymerization of 1-chloro-3-piperidine-2- propylacrylate with acrylic acid has been studied. The values of the copolymerization constants of CLPPAA (r1) and AA (r2) were calculated by graphical solution of the Feineman-Ross differential equation, which are 0.66 and 0.44, respectively. 2. The study of acute toxicity and antimicrobial activity of the synthesized copolymers based on 1-chloro-3-piperidine-2-propyl-acrylate with acrylic acid showed that the tested compounds, according to the classification of IHS 12.1.007-76, according to the parameters of acute toxicity, belong to the IV class of low-toxic substances, have antimicrobial activity against strains St. Epidermidis, E. coli и Bac. Subtilis.
References
| [1] | Панарин, Е.Ф. Полимерные лекарства и биологически активные вещества / Е.Ф. Панарин // Полимеры и медицина. – 2005. – № 1. – С. 20-24. |
| [2] | Ghorbanloo, M. pH sensitive hydrogel based acrylic acid for controlled drug release / M. Ghorbanloo, A. Heidary // Phys. Chem. Res. – 2017. – V. 5. – № 1. – P. 185-193. |
| [3] | Каморин Д.М., Ширшин К.В., Орехов Д.В., Сивохин А.П., Садиков А.Ю., Казанцев А.О., Панина Е.А. Радикальная сополимеризatsiя акриловой кислоты и метоксиполиэтиленгликольметакрилата в водном растворе. // Пластические массы. -2017. -№1-2. –С.6-8. |
| [4] | Прочухан Ю.А., Прочухан К.Ю., Идогова Я.В. Влияние минерализatsiи воды на реологические свойства полимера // Башкирск. химич. журн. – 2014. –Т.21. –№4. –С.80–83. |
| [5] | Hui H. Thermo- and pH-sensitive dendrimer derivatives with a shell of poly(N,N- dimethylaminoethylmethacrylate) and study of their controlled drug release behavior / H. Hui, F. Xiao-dong, C. Zhong-lin // Polymer. – 2005. - 46. – P. 9514–9522. |
| [6] | Xaperskaya LS, Medetbekova JM, Sarymzakova R.K. Synthesis of new biologicheski actin so-called on osnove n-zameshchenny gamma-piperidonov // Uspexi sovremennogo estestvoznaniya, 2016. - №9. - p. 38–42. |
| [7] | Safrokov AP, Terziyan T.V. Acrylic hydrocele acrylic and acrylic acid acetate, initsirovannoe persulfatom ammonia // Vysokomolek. soedinenie. Series B. - 2015. –T. 57. - № 5. - p. 338–345. |
| [8] | J. Jayabharathi, V. Thanikachalam, M. Padamavathy, M. Venkatesh Perumal, J. Fluoresc. 22 (2012) 269-279. https://doi.org/10.1007/s10895-011-0957-5. |
| [9] | M. Arockia doss, S. Savithiri, G. Rajarajan, V. Thanikachalam, C. Anbuselvan, Spectrochim. Acta Part A. 151 (2015) 773-784. https://doi.org/10.1016/j.saa.2015. 07.024. |
| [10] | Pulatova N.U., Maksumova O.S. Synthesis of polymers based on 1-chloro-3-piperidine-2-propyl methacrylate // 8 th International conference Science and society -Methods and problems ofpractical application. Vancouver, Canada 2019. -РР. 139 - 141. |
| [11] | Pulatova. N.U., Maksumova. O.S. Piperidine compounds synthesis and properties, ISBN 978-620-4-72608-3, Moldova ”LAP LAMBERT Academik Publishing” 2021.57 P. |
| [12] | Pulatova N.U., Maksumova O.S. Synthesis of esters based on piperidine // The scientific method. 10/Warszava, Poland 2017, 26 - 28 p. |
| [13] | N.U. Pulatova, O.S. Maksumova, Izabela Barszczewska Rybarek. Synthesis and polymerization processes of 1-chlorine-3- piperidine -2-propylmethacrylate monomer Technical science and innovation: Vol. 2021: Iss. 3, Article 4. Available at: https://uzjournals.edu.uz/btstu/vol2021/iss3/4. |
| [14] | Методические указания в Руководстве по экспериментальному (доклиническому) изучению новых фармакологических веществ. Под общей редакцией члена-корреспондента РАМН, профессора Р. У. Хабриева. Издание второе, переработанное и дополненное. -М.:ОАО «Издательство «Медицина», 2005. – 830 с. |
| [15] | Беленький М.Л. Элементы количественной оценки фармакологического эффекта. Л.: Медгиз, 1963. – 152 с. |
| [16] | Определение чувствительности микроорганизмов к антимикробным препаратам диско-диффузионным методом (методические указания)» № 012-3/0093, Ташкент, 2007. |
| [17] | Государственная Фармакопея, XI издание, выпуск 2. Москва: «Медицина», 1990. – С.210-215. |
| [18] | Государственная Фармакопея РУз, I издание, 2 часть, Ташкент 2021. – С.1451-1453. |










 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML



