-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Polymer Science
p-ISSN: 2163-1344 e-ISSN: 2163-1352
2022; 11(1): 7-17
doi:10.5923/j.ajps.20221101.02
Received: May 28, 2022; Accepted: Jun. 13, 2022; Published: Jun. 29, 2022

Synthesis, Characterization, and Use of the Dimer of (Nitrato-κ2 O, O′) Bis (2-(2,4-dinitrobenzyl) Pyridine κ-N) Silver(I) for the Decoration of Polyanilines with Metallic Silver
Jose Medina, Jaclyn McCasland, Jordan McBride, Roch Chan-Yu-King
University of Science and Arts of Oklahoma, Science Division, Grand Avenue and 17th Street, Chickasha, OK, USA
Correspondence to: Roch Chan-Yu-King, University of Science and Arts of Oklahoma, Science Division, Grand Avenue and 17th Street, Chickasha, OK, USA.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The reaction of the photochromic compound, 2-(2, 4-dinitrobenzyl) pyridine, with AgNO3 in methanol yields a coordination product that crystallizes out as the dimer of (Nitrato-κ2 O, O′) bis (2-(2,4-dinitrobenzyl) pyridine κ-N) silver (I). The organosilver is characterized by 1H-, 13C-NMR, HRMS, elemental analysis, cyclic voltammetry and X-Ray crystallography. On exposure to sun light, the dimer reveals to be devoid of photochromism. However, it can be used to decorate polyanilines (PANI) with metallic silver in a solventless/heterogeneous medium at high temperature or under homogenous condition in NMP solvent at room temperature. Upon decoration, the PANI-Ag composites are analysed via SEM, EDS, UV-Vis and IR. Four-probe measurements of the silver coated polyanilines provide electrical conductivities higher than those of the corresponding silver free parent PANI.
Keywords: X-Ray crystallography and ORTEP drawing of the dimer of (Nitrato-κ2 O, O′) bis (2-(2,4-dinitrobenzyl) pyridine κ-N) silver (I), Thermal/solid state or room temperature post-polymerization decoration of polyanilines with an organosilver (I) coordination compound, Derivative of 2-(2, 4-dinitrobenzyl) pyridine, Band gap of polyaniline-silver composites
Cite this paper: Jose Medina, Jaclyn McCasland, Jordan McBride, Roch Chan-Yu-King, Synthesis, Characterization, and Use of the Dimer of (Nitrato-κ2 O, O′) Bis (2-(2,4-dinitrobenzyl) Pyridine κ-N) Silver(I) for the Decoration of Polyanilines with Metallic Silver, American Journal of Polymer Science, Vol. 11 No. 1, 2022, pp. 7-17. doi: 10.5923/j.ajps.20221101.02.
Article Outline
1. Introduction
- Among the organic photochromic compounds, 2-(2, 4-dinitrobenzyl) pyridine (DNBP) is one of the most investigated [1]. When kept in the dark, this compound is sandy brown in colour which turns into deep blue on exposure to UV/sun light. The mechanism of this reversible photochromic reaction involves the formation of two tautomeric structures. They are generated through an initial intramolecular deprotonation of an acidic benzylic hydrogen by an oxygen of the NO2 group (Fig. 1) leading to the less stable "OH" tautomer which eventually rearranges to the more stable "NH" intermediate [2]. However, the potential participation of the N-pyridyl atom in photochromism (upon the formation of the tautomers), via an inter or intramolecular charge transfer to the electrophilic dinitrated benzylic ring has not been investigated. In addition, an inhibition of photochromism, through complexation of the N-atom with a transition metal cation has not been addressed. It is well documented that pyridine and its derivatives are often used as ligands for the complexation of transition metal cations in the formation of coordination compounds and the lone pair of nitrogen, in some instances, can transfer its charge to an oxidizing receptor [3]. To gain insight into the above-mentioned potential role of the N-pyridyl atom in charge transfer processes, we have reacted DNBP with silver nitrate and have unexepectedly isolated the dimer of (Nitrato-κ2 O, O') bis (2-(2,4-dinitrobenzyl) pyridine κ-N) silver(I) whose structure is shown in Fig. 2.
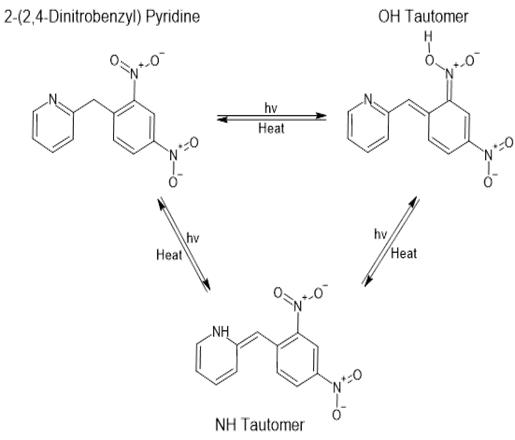 | Figure 1. Sun light induced tautomerism in 2-(2,4-dinitrobenzyl) pyridine resulting in photochromism |
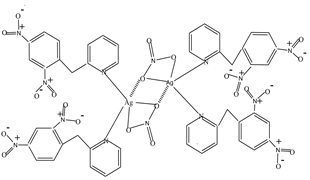 | Figure 2. Structure of the dimer of (Nitrato-κ 2 O, O') bis (2-(2,4-dinitrobenzyl) pyridine κ-N) silver(I). The dotted lines indicate bridges that bond the two monomers |
2. X-Ray Structure of the Dimer
- The dimer structure was determined by X-Ray crystallography (details of the crystallographic data on bond lengths, angles, H-bonds are attached in a separate file). A brief description of the multidimensional structure of the dimer is as follows: Fig. 3 (ORTEP view) shows two individual Ag(I) containing monomers with the bridging bonds left out for clarity. Each Ag(I) center (blue atom) adopts a highly distorted tetrahedron geometry and is bonded to two N-atoms (green atoms) from the DNBP ligands and two O-atoms (red atoms) from a bidentate (NO3-) ligand. Fig. 4 (ORTEP view) features the bridges (Ag1-O22 and Ag2-O21) linking the two monomers. The binuclear dimer is rather unique in that each metallic center participates in the formation of a relatively small (Ag1-O22-Ag2-O21) tetracyclic (parallelogram-like) ring in which two trivalently coordinated O-atoms (O22 and O21) are found. A rare but similar type of structure has been reported by Bowmaker et al. in a quinoline Ag(I) nitrate complex [4].
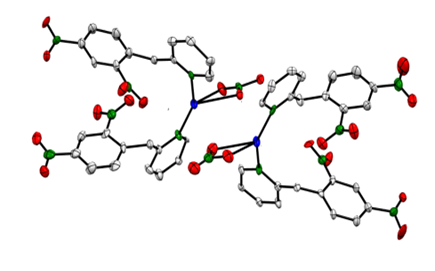 | Figure 3. ORTEP view of two monomers of (Nitrato-κ 2 O, O') bis (2-(2,4-dinitrobenzyl) pyridine κ-N) silver(I). Bridging bonds are not shown |
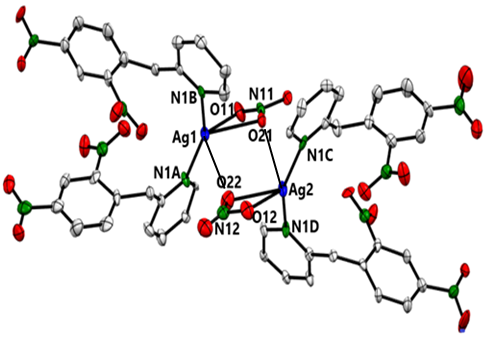 | Figure 4. ORTEP view of the dimer featuring bridging bonds (Ag1-O22 and Ag2-O21). C-atoms are in grey, O-atoms in red, and H-atoms are left out |
3. Experimental Section
3.1. Synthesis of the Dimer
- The DNBP ligand was prepared according to the procedure of Zaccek [6]. It was recrystallized and then reacted with silver nitrate according to the following procedure: a solution of 0.17g (1.00 mmol) of silver nitrate in 7.00 ml methanol was added dropwise to a solution of 0.26 g (1.00 mmol) of DNBP in 6.40 ml methanol. The resulting mixture was stirred at room temperature for 3h after which time it was left undisturbed. The solvent was allowed to partially evaporate for several days resulting in the formation of yellowish crystals which were collected and recrystallized in methanol. The product was filtered, washed with cold hexanes, and then vacuum dried to provide 0.280g (81% yield) of dimer. The yield is based on the amount of ligand as the limiting reactant in a reaction of a 1/2 stoichiometric (metal/ligand) ratio. Elemental analysis (Micro Analysis, Inc.) calculated for C48H36Ag2N14O22 (1376.64): C 41.88%, H 2.64%, N 14.24%; Found: C 41.88%, H 2.60%, N 14.16.
3.2. Spectral Analyses of the Dimer
- a) High resolution NMR data are collected with a Varian 300 MHZ broad band Gemini spectrometer: 13C-NMR (DMSO-d6), δ (ppm): 40.36 (sp3 C) and aromatic sp2 C’s: 120.01, 122.19, 123.67, 127.41, 134.60, 137.50, 140.36, 146.39, 148.8, 149.48, 157.37.1H-NMR (DMSO-d6), δ (ppm): 4.63 (s, CH2), 7.29 (m, 1H), 7.35 (d, J=7.9Hz, 1H), 7.81 (m, 2H), 8.44 (m, 1H), 8.50 (dd, J=8.2 Hz, J= 2.2 Hz, 1H), 8.76 (d, J=2.5 Hz, 1H). Both 13C- and 1H-NMR spectra resemble those of the DNBP molecule [7] except the ligand peaks associated with the dimer are all slightly shifted downfiled due to their complexation with the Ag(I) ion.b) FTIR spectra (400-4000 cm -1 range) and UV-Vis (300-900 nm range) of the parent PANI or its polymer-silver composites are recorded with the use of a Nicolet iS10 (Thermoscientific Corporation) and UV-2550 spectrometers (Shimadzu Co.), respectively.FTIR of the dimer (KBr pellet, cm-1): 3109.15(=C-H stretching), 3079.96 (=C-H stretching), 2923.55 (C-H stretching), 1603.47 (C=C stretching), 1568.47, 1525.87 (NO2 stretching), 1479.59, 1439.83, 1350.62 (stretching of NO3-), 1302.00 (stretching of NO3-), 1150.97, 1097.37, 1066.75, 1036.99, 1015.51, 913.50, 853.41(C-H bending), 836.56 (C-H bending), 819.68 (C-H bending), 807.93, 770.81, 740.88, 728.61, 723.07, 686.46, 650.61, 639.66, 611.27. c) High resolution mass spectral data were obtained with a Bruker 15T FT-ICR instrument. ESI-MS (DMSO solvent). Keeping in mind that Ag has two major isotopes whose natural abundances are almost in a 1:1 ratio, the two most intense peaks observed in the spectrum belong to the monomeric cation with m/z of 625.341 [M+] and 627.341 [M+ + 2]. Other significant peaks have m/z of 365.963 [M+ - (one ligand)], 367.962 [(M+ + 2) - one ligand], 260.056 [(one ligand) + H]+,166.946 [dinitrophenyl]+ and 164.946 [dinitrophenyl –(2 H’s)]+. The absence of any significant size/detectable dimer molecular ion peak is either due to its high instability under the energy intense mass spectrometry (MS) condition or prior to MS analysis, the dimer would have dissociated readily when dissolved in the polar DMSO solvent. Thus, only the monomeric ions and their fragmented peaks were observed.
3.3. Melting Point of the Dimer
- On exposure to sunlight for extending periods, the dimer did not exhibit photochromism. However, when heated, it melted at 126-128°C producing a yellowish melt at first followed by the gradual formation of spherical, lustrous silver particles around 135-138°C (inset in Fig. 5).
3.4. Cyclic Voltammetry of the Dimer
- The electrochemical data were collected using a CH-instrument (CHI 760D) potentiostat equipped with Electrochemical Analyzer software (version 15.03). The reference electrode is Ag/AgCl. The counter electrode is platinum based with surface area of 1x1 cm2. The dimer (3mg) was deposited on carbon cloth (1x1 cm2) functioning as the working electrode. The electrolyte and scan rate are 1M Na2SO4 and 10 mV/s, respectively. The cyclic voltammogram (Fig. 5) shows an oxidation and reduction peaks of silver at 0.522V and 0.292V, respectively.
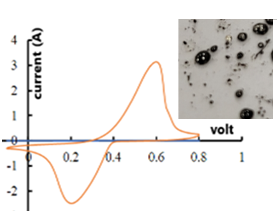 | Figure 5. Cyclic voltammogram of the dimer (scan rate 10mV/s). Inset: photograph of metallic silver particles formed upon heating the dimer |
3.5. Analyses of the Parent Polymer and Its Composites
- a) Electrical conductivity The conductivity of the parent ES and its silver composites was measured on pelletized powder, which was compressed with a hydraulic press at 700 MPa, resulting in a circular pellet of 13 mm in diameter with a thickness of about 0.95 mm. The pellets were then connected to a four point-probe device which is linked to a Lakeshore 120CS DC microcurrent source coupled to an auto ranging multimeter 175A (Keithley/Tektronix Co.) The current is passed through two adjacent contacts and the resulting voltage recorded. The electrical measurements were conducted on both sides of the pellet. The conductivities are determined via the Van Der Pauw method [8] and their values averaged out. b) MorphologySurface morphological characteristics of PANI composites are obtained via a SEM instrument (Zeiss Neon EsB operating at 5 KV) which is connected to an energy dispersive X-ray spectrometer (EDS) operating at 10-15 KV. Prior to SEM analyses, the composites were briefly sonicated in water and a sample of the suspension was retrieved via pipet and then air dried.
3.6. Typical Procedures for the Preparation of the Parent ES and EB
- Into a 250-mL beaker, containing a magnetic stir bar, was placed a solution of 1.56g (16.0 mmol) freshy distilled aniline in 120 mL 1M HCl. To this stirred solution was added 4mg of SWNT used as template to guide PANI nanofiber growth [9], [10]. Then a solution of 0.73g (3.19 mmol) of ammonium peroxydisulfate (APS) in 80 mL 1M HCl was added to the anilinium chloride solution in small portions. The resulting mixture was stirred overnight, suction filtered to yield a deep green precipitate which was washed with copious amounts of water, and then acetone until the washings became colourless. The ES was high vacuum dried for 5 h at 50°C. To prepare the corresponding EB, the as-prepared/dried ES was placed in a 1L volumetric flask containing 0.10M NH3 (aq) The mixture was stirred overnight, and suction filtered. The cake was washed with abundant amounts of water, and then acetone. The deep blue EB was collected and then high vacuum dried for 6 h at 50°C.
3.7. Typical Procedures for the Decoration of EB and Its Post Protonic Doping to ES-Ag (6%) Composite
- All experiments were repeated at least twice. It is worth noting that in this work the post polymerization metal decoration of the PANI starts invariably with EB. Had ES and the dimer been used as reagents, there could be potential precipitation of AgCl since the chloride ions from the ES and the silver (I) ion in the dimer could combine to form the precipitate.a) Under thermal condition in a heterogenous medium.Into a mortar were placed 200 mg of EB and 81 mg dimer of which 12.7 mg is Ag (the dimer contributes a theoretical 6% Ag by mass in the final composite). The mixture was pulverized in the mortar with a pestle. The powder was transferred into a test tube and then heated in a sand bath up to 145°C at which point the heat bath was removed. The mixture was allowed to cool and then diluted in 100 mL DI water with stirring. The top portion of the liquid supernatant was discarded, the remaining mixture centrifuged and the water was discarded. This step was repeated three more times. Acetone was then added to the solid residue under stirring. The mixture was centrifuged, and the solvent was discarded. Additional washings with acetone were repeated until the solvent became colourless. The resulting solid, obtained after centrifugation, was dried under high vacuum at 60°C for 4h. To convert the EB-Ag to the conducting ES-Ag, the composite was placed in 500 mL 1M HCl (aq) and the suspension was stirred overnight. The mixture was suction filtered, sequentially washed with water and acetone and then dried in high vacuum for 6h at 60°C.b) At room temperature in a homogeneous medium. Into a 50 ml Erlenmeyer flask containing 6.10g NMP was added, in small portions with stirring (over the course of 60 min) 200mg of pulverized EB. The mixture was stirred for 4 hours and a solution of 81 mg of dimer in 0.20g NMP was added in small volumes over a 30-min period. The resulting solution was further stirred overnight after which time, it was transferred to a beaker containing 300 ml DI water. This mixture was allowed to settle and about 2/3 of the top deep blue supernatant was discarded. The remaining suspension was centrifuged, and the solid residue was sequentially washed and centrifuged two more times with DI water. It was then repeatedly washed with acetone and centrifuged until the solvent became colourless. The composite was dried in high vacuum for 6 h (50°C). The conversion of the isolated EB-Ag to ES-Ag composite was conducted in the same manner as described above.
4. The Common Oxidation States of PANI
- Polyaniline (PANI) is one of the most studied, environmentally stable, and intrinsically conducting polymers. The three commonly cited oxidation states of PANI are Pernigraniline, Emeraldine and Leucoemeraldine (Fig. 6). They could be present either in their protonated (salt) or deprotonated (base) state. For a practical standpoint, the most useful form of PANI is Emeraldine because its preparation is straight forward, and it can be easily and reversibly switched from the insulating emeraldine base (EB) to the conducting emeraldine salt (ES) state, by protonation with an acid such as HCl (aq). The ES, in turn, can be dedoped/deprotonted with a base (NH3) back to the insulating EB (Fig. 7).
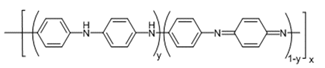 | Figure 6. Base forms of Polyaniline: a) Pernigraniline (PB), y =0 b) Emeraldine (EB), y = 0.5 and c) Leucoemeraldine (LB), y =1. x is the number of repeat units |
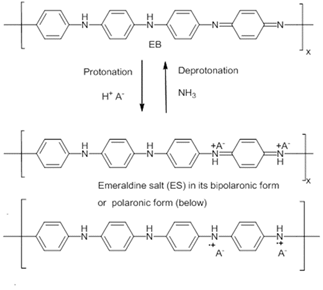 | Figure 7. Process of protonation/doping of EB to form ES and deprotonation/dedoping of ES to form EB |
5. Results and Discussion
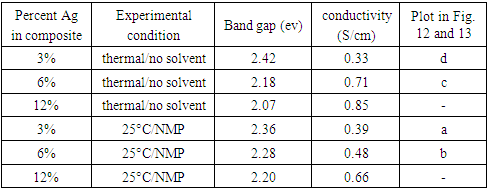
- A series of silver decoration experiments of PANI with the dimer was conducted with EB either in solid state at high temperature or in NMP at ambient temperature. Assuming the reduction of Ag(I) to Ag metal is quantitative, the theorical mass % Ag in the final composites are 3%, 6% and 12%.
5.1. SEM Surface Analyses of PANI-Ag Composites
- The decoration of PANI with metallic silver is conspicuous as revealed by SEM analyses. For example, Fig. 8a of a sample of EB-Ag (3%), (obtained under the stirred condition with NMP as solvent) shows the presence of unevenly dispersed metal clusters whose individual (semi) spherical metal particles are less than 100nm in diameters (Fig. 8b). They are coated onto the EB nanofibers of <100 nm in diameters. Semi quantitative elemental determination via EDS confirms the presence silver (Fig. 8c) when a larger size cluster (black dot on Fig. 8d) was analyzed. When a sample of EB-Ag was doped with 1M HCl, the resulting ES-Ag (3%) has metallic particles with sizes similar to those of the previously described dedoped EB counterparts (Fig. 8e). However, when the experiments were conducted with higher amounts of dimers, the as-produced PANI-Ag cluster morphology departs drastically from the above-described semi spherical structure, regardless of the experimental protocol used. The deposited Ag(s) exhibit various shapes and sizes with dimensions ranging from nano- to micrometer(s). For example, Fig. 8f displays an EB-Ag(6%) sample prepared in NMP at room temperature. The pebble like Ag(s) aggregates in Fig. 8g belongs to a sample of EB-Ag (6%) produced under thermal/solventless condition. And Fig. 8h features larger crystallites of an ES-Ag (12%) composite prepared in NMP solvent at room temperature.
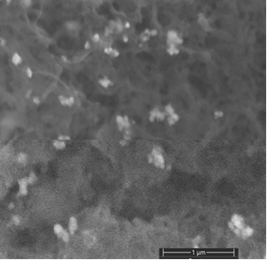 | Figure 8a. EB-Ag (3%) composite isolated from NMP as solvent at room temperature (scale bar: 1μm) |
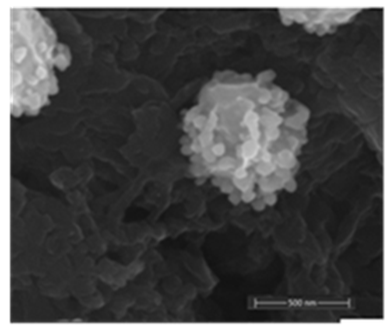 | Figure 8b. EB- Ag (3%) composite showing a semi spherical Ag cluster composed of metallic particles of <100 nm in diameters (scale bar: 500 nm) |
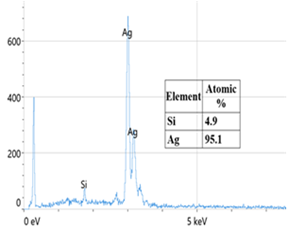 | Figure 8c. EDS of an EB-Ag omposite confirming metallic silver deposited on EB. The presence of Si is from the silicon wafer used as substrate onto which the EB-Ag is deposited |
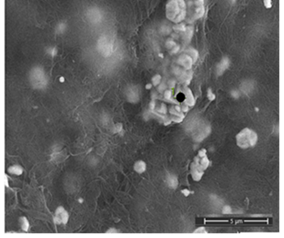 | Figure 8d. EB- Ag (3%) composite with a large Ag cluster (scale bar: 1 μm) |
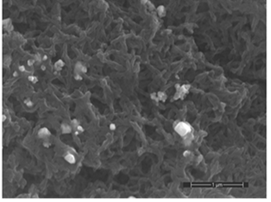 | Figure 8e. ES-Ag (3%) composite with Ag deposited on nanofibers of PANI prepared from thermal/solventless condition (scale bar: 1μm) |
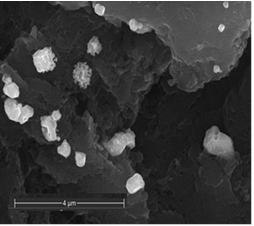 | Figure 8f. EB-Ag (6%) composite isolated from NMP as solvent at room temperature (scale bar: 4 μm) |
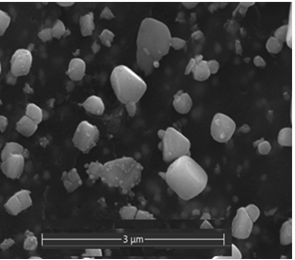 | Figure 8g. EB-Ag (6%) composite prepared under solventless/thermal condition (scale bar: 3 μm) |
 | Figure 8h. ES-Ag (12%) composite prepared in NMP/room temperature (scale bar: 1 μm) |
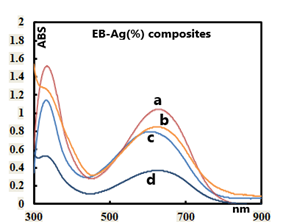
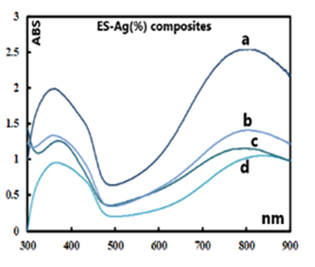
5.2. UV-VIS Analyses of the PANI-Ag Composites
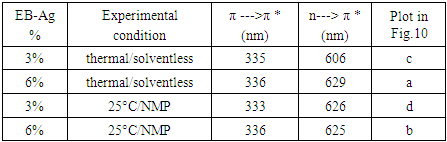
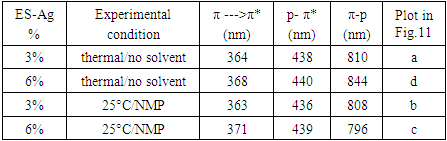
- The UV-Vis spectra of the parent/undecorated EB (Fig 9) displays the two typical absorptions bands: one at 328 nm which is assigned to the π-π * transition in the benzenoid ring, and the other at 635 nm which is associated with the excitation transition (“n-π*”) in the quinoid rings [20].
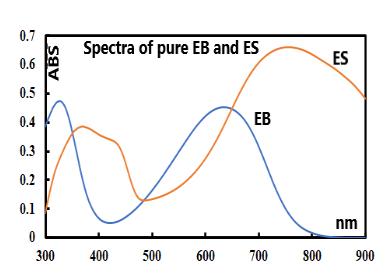 | Figure 9. UV-vis spectra of the undecorated parent EB and ES |
|
|
5.3. Determination of Band Gap
- The electric conductivity of the intrinsically conducting polymers are obtained via the four-probe technique and their band gaps (BG) are determined using the Tauc equation: αhv=A(hv-Eg)n where α is the absorbance, hv the incident photon energy, A is the BG tailing coefficient (a constant nearly independent of the photon energy parameter) and Eg the optical BG. The exponent n can take values of 0.5, 1.5, 2 or higher depending on the nature of the transitions, i.e., whether they are allowed or forbidden and direct or indirect. If the transition is allowed and direct, the n value is 0.5. An n value of 1.5 indicates a direct but forbidden transition whereas a value of 2 is associated with an allowed but indirect transition [22]. The photon energy hv (in ev) can be expressed as a function of the incident light wavelength and written as 1240/λ, with λ in nm. In this study, the energy (1240/λ) is plotted against (αhv)0.5 which provided a straight line fit implying a direct and allowed transition. The BG is obtained by extrapolating the straight-line portion of the graph onto the hv axis where α=0. Representative plots for the BG determination are given in Fig. 12 and 13.
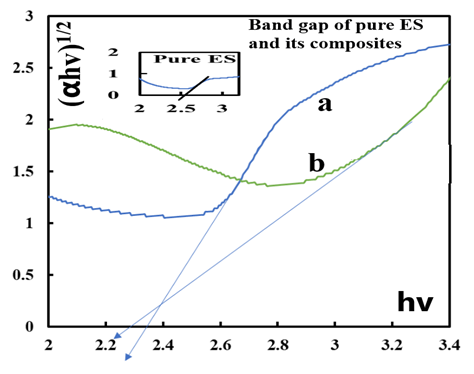 | Figure 12. Tauc plots for the determination of band gap values of pure ES (inset) and ES-Ag (%) composites prepared at room temperature in NMP a: (3%) ; b: (6%) |
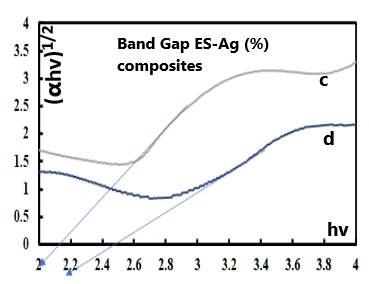 | Figure 13. Tauc plots for the determination of band gap values of ES-Ag (%) composites prepared under solventless condition at high temperature: c: (6%); d: (3%) |
|
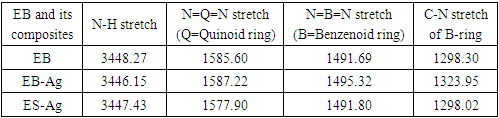
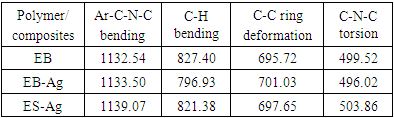
5.4. IR Analyses
- Representative IR spectra of EB, EB-Ag and ES-Ag are shown in Fig. 14, 15 and 16. Their vibrations (cm-1) are as follows: EB: 3448.27, 1585.60, 1491.69, 1409.77, 1378.10, 1298.30, 1267.13, 1159.20, 1132.54, 954.37, 854.37, 827.40 695.72, 499.52.EB-Ag: 3446.15, 1587.22, 1495.32, 1384.01, 1323.95, 1286.01, 1133.50, 1123.73, 796.93, 701.03, 496.02.ES-Ag: 3447.43, 1577.90, 1491.80, 1383.41, 1298.02, 1139.07, 821.38, 797.94, 697.65, 599.30, 503.86.
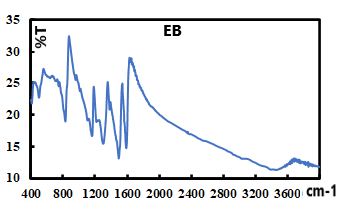 | Figure 14. IR spectrum of pure EB |
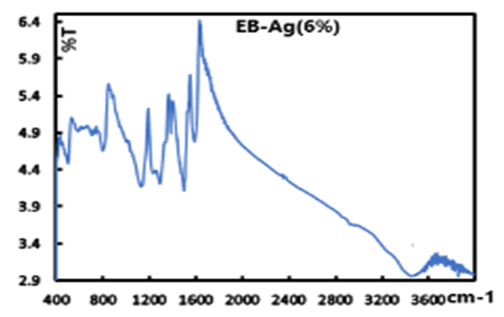 | Figure 15. IR spectrum of EB-Ag (6%) prepared from thermal/solventless condition |
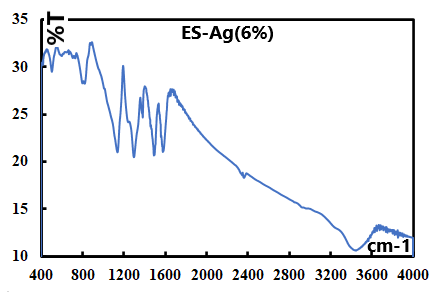 | Figure 16. IR spectrum of ES-Ag (6%) prepared in thermal/soventless condition by doping the corresponding EB-Ag(6%) with 1M HCl |
|
|
6. Conclusions
- We have synthesized and characterized the dimer of (Nitrato-κ2 O, O′) bis (2-(2,4-dinitrobenzyl) pyridine κ-N) silver (I). Owing to the redox properties of this organosilver compound, we showed that it can be used to decorate polyanilines under thermal/solventless condition or at room temperature in NMP solvent. Both procedures yield silver decorated PANI which were characterized by UV-Vis, IR spectroscopy and SEM. Surface analyses of the composites via SE micographs revealed that at low silver concentration, the metal is deposited on the PANI matrix as (semi) spherical clusters composed of particles with average diameters in the nanometer range. At higher concentrations, the particles tend to aggregate forming larger structures of various shapes with dimensions ranging from nano- to micrometers. It is found that the conductivity of the composites increases with increasing loading of silver and is moderately above that of the undecorated PANI.
ACKNOWLEDGEMENTS
- The authors are grateful to the Foundation of the University of Science and Arts of Oklahoma for its partial financial contribution to the project through the Gladys Anderson Emerson research fund. They acknowledge the help and thank Dr. Douglass Powell of the University of Oklahoma for collecting X-Ray crystallographic data and for the determination of the dimer structure. Dr. Powell thanks the National Science Foundation for a grant (CHE-1726630) and funds from the University of Oklahoma for the purchase of the X-ray diffractometer and computers.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML