-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Polymer Science
p-ISSN: 2163-1344 e-ISSN: 2163-1352
2019; 9(1): 1-9
doi:10.5923/j.ajps.20190901.01

Nanostructures Obtaining on the Basis of Bombyx Mori Chitosan Hydroxyapatite
Vokhidova N. R., Ergashev K. H., Yugay S. M., Rashidova S. Sh.
Institute of Polymer Chemistry and Physics of Academy of Sciences of Uzbekistan, Tashkent, Uzbekistan
Correspondence to: Vokhidova N. R., Institute of Polymer Chemistry and Physics of Academy of Sciences of Uzbekistan, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Composites of Bombyx mori chitosan with hydroxyapatite (Ca10(PO4)6(OH)2) have been obtained. Obtained chitosan - hydroxyapatite composites were studied by elemental analysis, conductometric titration and XRD, FTIR, UV and AFM methods. It was shown that interaction takes place between functional groups of chitosan and hydroxyapatite by hydrogen bonds. It was revealed that nanostructure composites with minimal sizes from 2 to 15 nm are forming at the selected synthesis conditions. Methods of composites regulation have been elaborated. The obtained composites are of an interest in production of biomaterials for traumatology, dentistry and veterinary.
Keywords: Bombyx mori chitosan, Hydroxyapatite, Nanocomposite
Cite this paper: Vokhidova N. R., Ergashev K. H., Yugay S. M., Rashidova S. Sh., Nanostructures Obtaining on the Basis of Bombyx Mori Chitosan Hydroxyapatite, American Journal of Polymer Science, Vol. 9 No. 1, 2019, pp. 1-9. doi: 10.5923/j.ajps.20190901.01.
1. Introduction
- Biopolymer chitosan (CS) and its derivatives are of a great interest for practical application due to their valuable properties such as biocompatibility, biodegradation, bactericide and others. The special attention is devoted to composites on the base of biopolymer including CS and hydroxyapatite (HA) with biological active properties, represented a new class of preparations for osteoporoses therapy and substitution of human bone tissue [1-6]. CS scaffolds alone cannot imitate all properties of a natural bone. Composite materials with CS can mimic all properties of a bone. Calcium phosphate materials are osteoconductive and mimic the inorganic portion of natural bone, while CS/HA composite materials were proved to be promising in imitation as the organic and inorganic portion of a natural bone. Several studies have been conducted with CS/HA composite materials for bone tissue engineering [7-9]. Polymers like СS have a higher degradation rate in compares on to bioceramics. Incorporation of HA into a СS polymer matrix has been shown to increase osteoconductivity and biodegradability with significant enhancement of mechanical strength [10]. Calcium phosphate compounds are of interest in the field of bone tissue engineering. HA is one of the most stable forms of calcium phosphate and it occurs in the bone as a major component (60 to 65%) [11]. Chitosan’s primary attractive features such as biocompatibility, biodegradability, flexibility, adhesiveness and anti-infectivity, make it as a feasible wound healing agent and the best polymeric matrix for HA ceramic [12].Recently, an interesting bio-inspired route for the design of composite materials containing CS and nanosized HA has been reported [13]. The authors suggested that a biomimetic approach should enable both the architecture (the structure) and the chemistry (the composition) of synthetic biomaterials to be controlled.The method consisted in of the formation of dicalciumphosphate dihydrate (DCPD) (CaHPO4*2H2O) in bulk CS acidic solution and their co-precipitation by gradually increasing the pH of the reaction medium by the dropwise addition of NaOH solution. It should be noted that all of the above mentioned approaches lead to a particular type of CS/HA composite material with a distinctive structure and properties. By a similar way, Peсa et al. [14] dispersed a commercial DCPD powder in a CS solution in diluted citric acid. The resulting viscous slurry was transformed at room temperature into a CS/Ca-deficient apatite/composite being poured into NaOH solution. DCPD is a well-known precursor of apatite and in different aqueous solutions can be hydrolyzed into octacalcium phosphate or apatite. Literature [1, 15-19] reported the FTIR spectra, X-ray powder diffraction (XRD) of apatite, CS/HA and their respective composites. Ca2+-ions appear on the terminated surface of HA crystals, which have coordination number of seven and are strictly held in the structure. Therefore, there is a possibility to form coordination bonds between the -NH2 of CS and Ca2+ of HA [5, 21, 22].The aim of this investigation is to investigate the composition and structure of Bombyx mori CS composites with HA.
2. Materials and Methods
- For obtaining of HA composites of Bombyx mori CS with molecular weight 280000 and degree of deacylation 86%, CaCl2 and KH2PO4 of “chemically pure” type have been used. Obtained samples were investigated by the elemental analysis.Samples were dried until constant mass using a freeze drier ALPHA 1-2 LD plus (Germany).The molecular weight of the polymer was measured by viscometry [23]. The degree of chitosan deacetylation and content of free (non-bonded) amino groups of CS in CS-HA was determined by conductometric titration. Final, total nitrogen content in chitosan was estimated by the Dumas method, using sample combustion in a quartz tube under carbon dioxide [24].UV spectra of cobalt acetate and chitosan with cobalt ware solutions were recorded on a Specord 210 UV-Vis spectrophotometer in the region 190-1000 nm with the use of 1 cm quartz cuvettes. The scanning rate was 5 nm/s.FTIR spectra were obtained with a SHIMADZU FTIR spectrometer. Samples were prepared in the form of KBr discs. Spectra were recorded over the range 4000-400 cm-1 at 4 cm-1 resolution.XRD investigation was carried out using a Multi Plot in the following conditions: Condition X-ray Tube: Cu (1.54060 A) Voltage: 30.0 kV Current: 30.0 mA ScanRange: 4.0000<->80.0000 deg Step Size: 0.0200 deg Count Time: 0.30 sec Slit DS: 1.00 deg SS: 1.00 deg RS:0.30 mm.Morphology of nanostructured film systems were studied on an atomic-force microscope AFM Agilent 5500 (USA) at room temperature. Silicon cantilevers with rigidity 9.5 N/m with frequency 145 kHz were used. Maximum field of scanning at AFM by Х, Y was 15x15μm2, by Z – 1 μm.
3. Results
- Compositions were obtained by addition in 2% solution of CS in acetic acid water solution of CaCl2 and KH2PO4 at a ratio Ca/P = 1.67. Ratio of chitosan and Ca2+-ions (CS:Ca2+) varied in intervals of 20:80; 50:50 and 80:20 (Table 1).
|
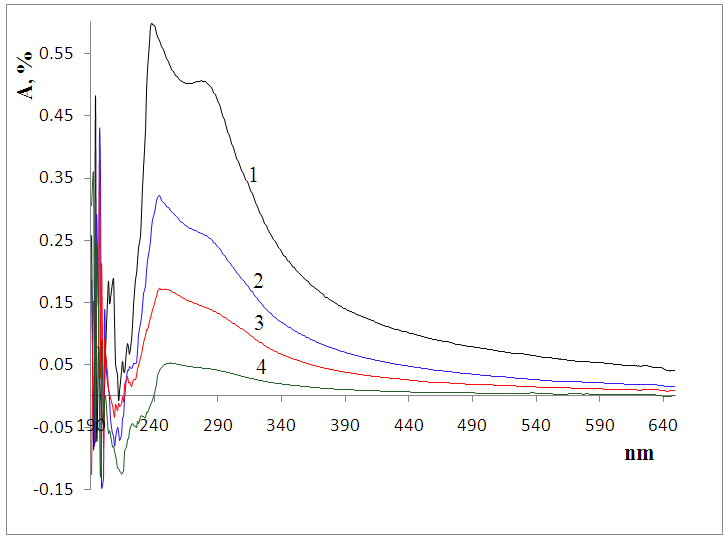 | Figure 1. UV spectra of Bombyx mori CS (1) and mixtures CS:Са2+/80:20 (2); 50:50 (3) and 20:80 (4) |
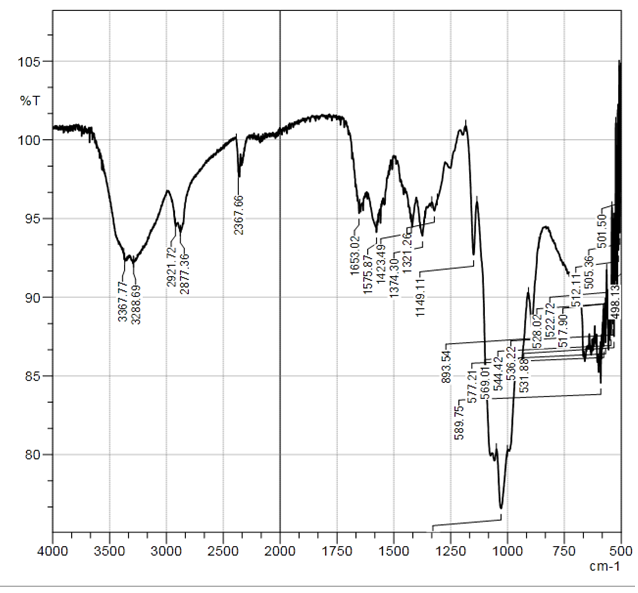 | Figure 2. Curve of FTIR of Bombyx mori CS sample |
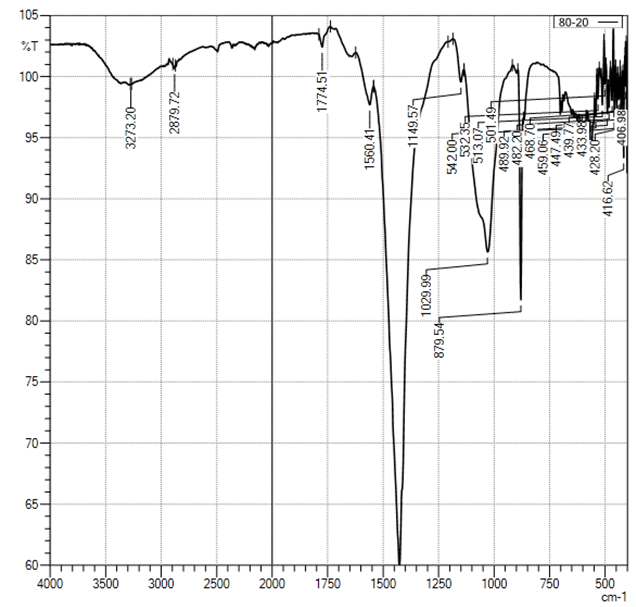 | Figure 3. Curve of FTIR of sample CS-Ca2+/80:20 |
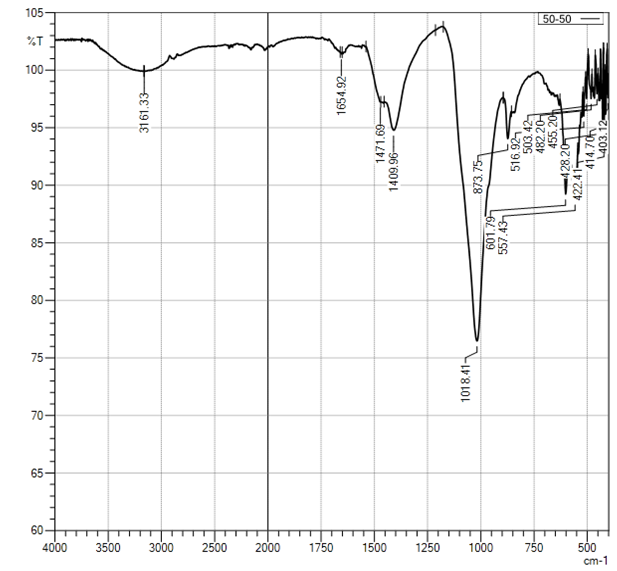 | Figure 4. Curve of FTIR of sample CS-Ca2+/50:50 |
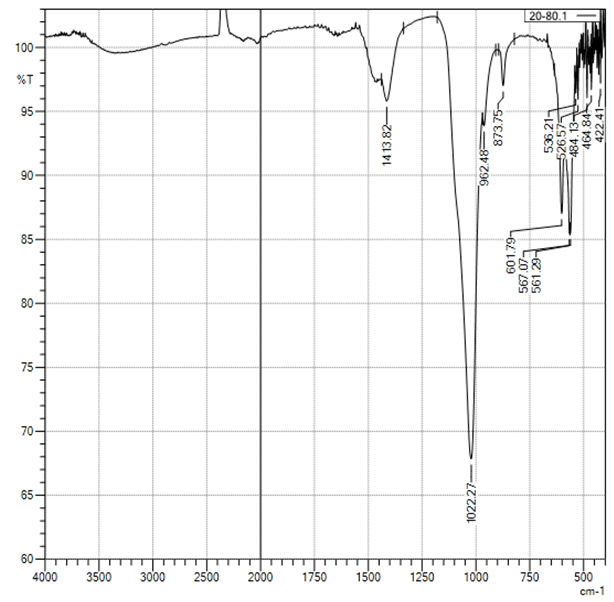 | Figure 5. Curve of FTIR of sampleCS-Ca2+/20:80 |
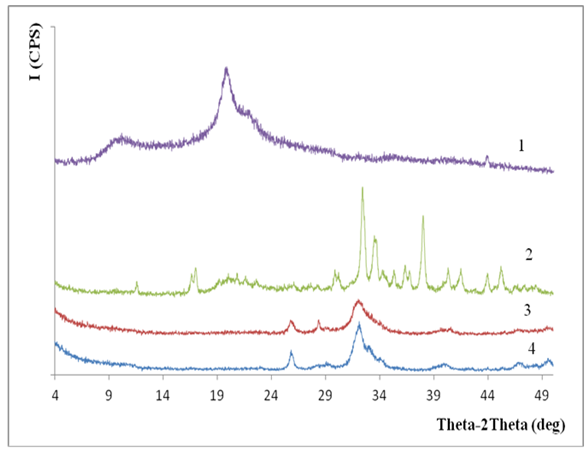 | Figure 6. X-ray diffraction of samples Bombyx mori CS (1), CS:Са2+ /80:20 (2), 50:50 (3), and 20:80 (4) |
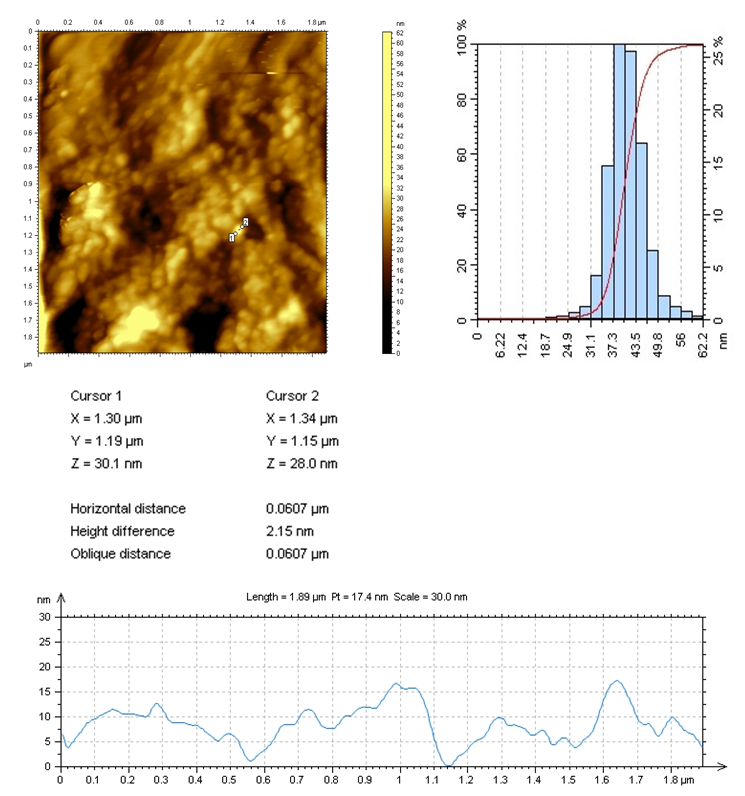 | Figure 7. AFM image of CS:Са2+/20:80 |
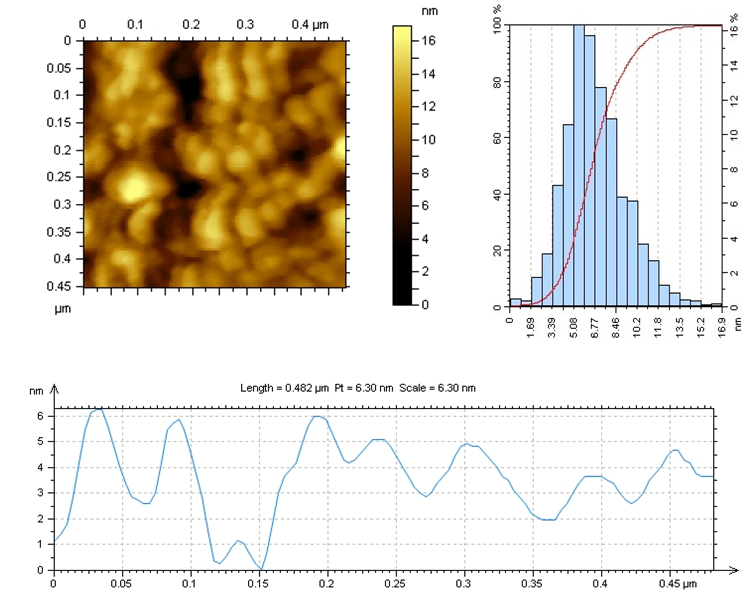 | Figure 8. AFM-image of CS:Са2+/50:50 |
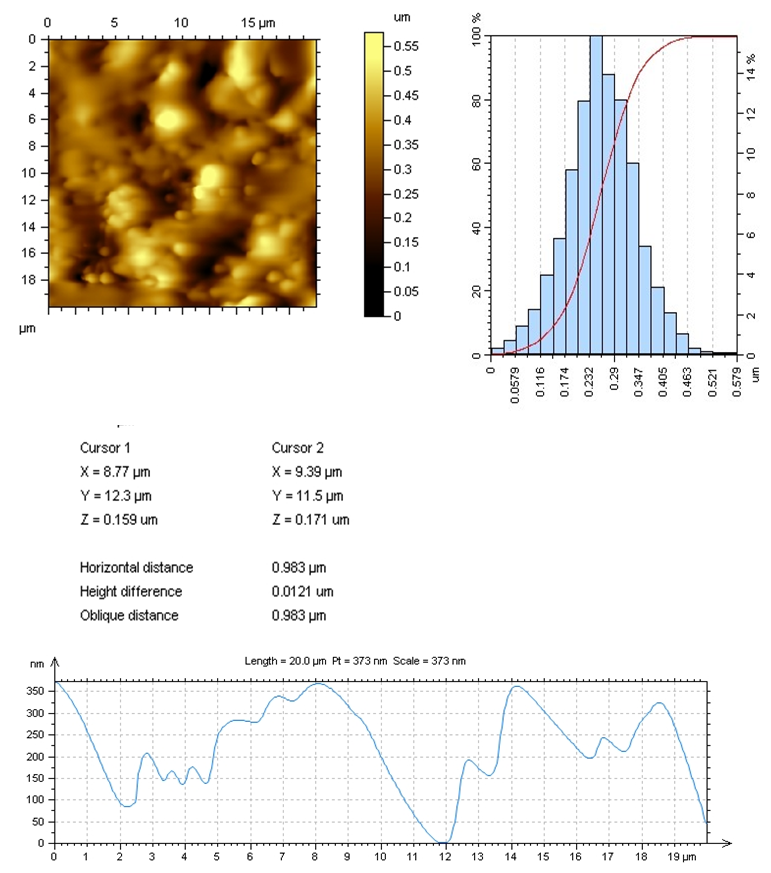 | Figure 9. AFM-image of CS:Са2+/80:20 |
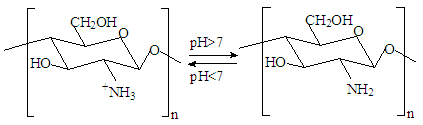 In the selected conditions for obtaining the composites of CS with Са2+, рН of reaction system рН<7 prevents interaction of Са2+ with NH3+. The results of conductometric titration, UV and FTIR investigations shown that interaction of chitosan macromolecule with hydroxyapatite molecule occurs due to hydrogen bonds of CS and НАfunctional groups.
In the selected conditions for obtaining the composites of CS with Са2+, рН of reaction system рН<7 prevents interaction of Са2+ with NH3+. The results of conductometric titration, UV and FTIR investigations shown that interaction of chitosan macromolecule with hydroxyapatite molecule occurs due to hydrogen bonds of CS and НАfunctional groups.4. Conclusions
- Composites of Bombyx mori chitosan with hydroxyapatite with content of Ca2+-ions till 17.5% have been obtained. It was found that, at the selected synthesis conditions, nanostructure composites with minimal sizes from 2 to 15 nm obtained.It was established that the interaction occurs between the functional groups of chitosan and hydroxyapatite by hydrogen bonds. It was shown that the introduction of hydroxyapatite into chitosan macromolecule leads to formation of insoluble composites. Low soluble composites CS-Ca2+ were obtained in increasing of Ca2+-ions content in the system. They are of interest of the biomaterials obtaining for traumatology, dentistry and veterinary.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML