-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Polymer Science
p-ISSN: 2163-1344 e-ISSN: 2163-1352
2018; 8(2): 23-28
doi:10.5923/j.ajps.20180802.01

Preparation and Characterization of Polyisocyanurate Modified Polyimide Foam
Ganiyu K. Latinwo1, Samuel E. Agarry1, Bukola K. Olopade1, Vincent E. Efeovbokhan2, Jimoh O. Hamed3
1Department of Chemical Engineering, Ladoke Akintola University of Technology, Ogbomoso, Nigeria
2Department of Chemical Engineering, Covenant University, Ota, Nigeria
3African Regional Centre for Space Science and Technology Education in English, Obafemi Awolowo University, Ile-Ife, Nigeria
Correspondence to: Ganiyu K. Latinwo, Department of Chemical Engineering, Ladoke Akintola University of Technology, Ogbomoso, Nigeria.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Poly (isocyanurate-imide)s (PIR-PI)s foams have been prepared from the reaction of polyimide (PI) and polyisocyanurate (PIR) prepolymers with different weight ratios of PIRs. The synthesis involve a two stage reaction process, in which firstly, a polyamic acid was produced from a polycondensation of pyromellitic dianhydride (PMDA) and 4,4’-oxydianiline (ODA) in the presence of isoquinoline (IQ), benzoic acid and dimethyl formamide (DMF). The polyamic acid was thermally imidized at 190°C to produce the PI precursor which was reacted with PIR prepolymer produced from the trimerization of 6.3 equivalents isocyanate and 1 equivalent polyol with 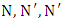 -tris (3-dimethylaminopropyl) hexahydro-s-triazine as catalyst. The PIR-PIs were characterized by infrared spectroscopy, thermal gravimetric (TGA), differential thermogravimetric (DTG), and solubility analyses. The PIR-PI foams were soluble in polar aprotic solvent only on heating, soluble in H2SO4 at room temperature, but insoluble in acetone. The PIR-PI foams showed excellent thermal stabilities. The temperature of T5% ranged from 328 – 350°C and the residual weights (Rw) were more than 40% at 450°C. The study revealed that presence of phenyl groups and imide structures in the PIR-PI backbone can improve thermal stability.
-tris (3-dimethylaminopropyl) hexahydro-s-triazine as catalyst. The PIR-PIs were characterized by infrared spectroscopy, thermal gravimetric (TGA), differential thermogravimetric (DTG), and solubility analyses. The PIR-PI foams were soluble in polar aprotic solvent only on heating, soluble in H2SO4 at room temperature, but insoluble in acetone. The PIR-PI foams showed excellent thermal stabilities. The temperature of T5% ranged from 328 – 350°C and the residual weights (Rw) were more than 40% at 450°C. The study revealed that presence of phenyl groups and imide structures in the PIR-PI backbone can improve thermal stability.
Keywords: Poly(isocyanurate-imide) foam, Solubility, Thermal properties, Spectroscopy
Cite this paper: Ganiyu K. Latinwo, Samuel E. Agarry, Bukola K. Olopade, Vincent E. Efeovbokhan, Jimoh O. Hamed, Preparation and Characterization of Polyisocyanurate Modified Polyimide Foam, American Journal of Polymer Science, Vol. 8 No. 2, 2018, pp. 23-28. doi: 10.5923/j.ajps.20180802.01.
Article Outline
1. Introduction
- Polyimides (PIs), are high performance heterocyclic polymers containing imide rings. These polymers possess outstanding thermo-oxidative, mechanical, insulating, and high chemical resistance characteristics and they have been widely applied in complex engineering applications in the electronics, electrical, automotive and aerospace industries [1-5]. PI is able to exhibit these properties due to the benzene and rigid imide ring structures in the polymer chain backbone. These structures also make the polymer to be insoluble, infusible and very difficult to process. In many of its applications, PI is deployed as film, fiber, foam or coatings. The resulting films or foams, in some cases are brittle [6, 7]. PI foams present one of the best suited materials for aviation and spacecraft because of the need for high strength-to-weight ratio and high quality materials in these ultra-complex engineering objects. Both the rigid and the flexible types of PI foams have been reported. Rigid PI foams have been made mostly from the reaction of aromatic dianhydrides and isocyanates [4, 8-11], but some have been made as TEEK foam by NASA [12, 13] and RohacellTM, PMI foams [13, 14] as well. Some US patents [15, 16] together with Yan et al and Tian et al [4, 11] have reported methods for producing flexible PI foam. It is interesting to note that all these foams suffer from deficiencies ranging from complexity in processing, low heat resistance, to insolubility, limiting their applications. It is well known that PI with inherent high thermal stability can be made easily processable using a number of efficient methods among which are incorporating flexible groups in the chain backbone to reduce chain stiffness, introduction of bulky pendent groups into the chain helping to hinder molecular packing for ease of crystallization, or synthesis of polymer blends. Exploring the polymer blends pathway has potential for synergistic contributions from the components, to the final polymer article, to produce a combined effect which is greater than provided by the separate materials. This was the factor for the improved thermal properties exhibited by polyetherimides, polyesterimides, polyamideimides and poly (urethane-imide)s as reported by [17-25]. The high thermal stability of the imides has been infused in the otherwise less thermally stable polymers. Polyisocyanurates (PIRs) are highly heat resistant, elastic, segmented polymers. PIR foam can be made by the trimerization and simultaneous blowing of isocyanate-terminated prepolymers made from approximately between 6 - 30 order magnitude equivalents of an isocyanate and 1 order equivalent of a polyol [26]. Due to its high heat resistant property PIR can be blended with PI to enhance the processability of the latter without diminishing its thermal stability in application requiring cellular foam structures. While different synthetic methodologies are available to enhance both thermal stability and solvent resistance of polyurethanes (PU)s by blending with PIs, there is only a handful of published data regarding the properties of PI affected by the PUs. Zuo et al. investigated the thermal stabilities and solvent-resistances of a series of poly (urethane-imide) prepared from the reaction of phenol end capped polyurethanes with poly(amic acid) precursors [27]. Zuo and Takeichi, and Iyer et al have carried out different studies on poly(hydroxyimide)s formed from the reaction of hydroxyl terminated diamines or dianhydrides with NCO-terminated polyurethanes [28, 29, 30]. Poly (urethane-imide)s containing oxazolidone rings was prepared by Radhakrishnan et al [31] from the crosslinked reaction of epoxy with imide structure and NCO-terminated polyurethanes. Yeganeh and co-workers have prepared poly (urethane-imide)s from reactions between hydroxyl-terminated poly (urethane-imide)s and polyisocyanate [23] and between epoxy containing polyurethanes and poly (amic acid) [32]. Sun et al [3] studied the fire resistance behavior of isocyanate-based polyimide (PIF) foam prepared from refilled 3,3’,4,4’-benzophenone tetracarboxylic acid dianhydride (BTDA) and polymethylene polyphenylene isocyanate (PAPI) by varying the polyimide proportion of the PIF foam. The study revealed that increase in polyimide proportion resulting from increase in refilled BTDA dosage remarkably improved the fire resistance of the foam reduce generation of ureido.In the present study, two series of poly (isocyanurate-imide) (PIR-PI) foams were prepared by the reaction of isocyanate-terminated PIR prepolymers, PI prepolymers, and deionized water as the blowing agent. The study focused on comparing properties of the resulting PIR-PI foams at different PIR prepolymer compositions. A model PIR foam was also prepared. The chemical structure, thermal stabilities, and processabilities of the foams were investigated.
2. Experimental
2.1. Materials
- Polycaprolactone polyol, CAPA (Mn ca. 4500) was purchased from Bristol Scientific and before use was dried at 90°C in vacuum for 22 h. 1,6 hexamethylene diisocyanate (HDI) purchased from Eurostar Scientific, UK, was purified by vacuum distillation.
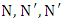 -tris (3-dimethylaminopropyl) hexahydro-s-triazine, m-cresol, methanol, dimethyl formamide (DMF), pyromellitic dianhydride (97%), 4,4’-oxydianiline (97%), and benzoic acid were purchased from Sigma-Aldrich. Deionized water was purchased from Ola Oluwa Gas Nigeria Limited.
-tris (3-dimethylaminopropyl) hexahydro-s-triazine, m-cresol, methanol, dimethyl formamide (DMF), pyromellitic dianhydride (97%), 4,4’-oxydianiline (97%), and benzoic acid were purchased from Sigma-Aldrich. Deionized water was purchased from Ola Oluwa Gas Nigeria Limited. 2.2. Measurements
- The infrared spectra of the rigid PI foams were carried out with Nexus-470 FTIR spectroscopy to characterize the chemical structures of the foams. The FTIR performed falls within the spectra range of 4000 and 400 cm-1, resolution is 4 cm-1 and apodization is triangular. Thermal gravimetric analysis (TGA) and differential thermo gravimetric analysis (DTG) were performed at heating rates of 10°C/min in nitrogen atmospheres via a thermal analysis instrument NETZSCH STA TGA-409C.
2.3. Synthesis
2.3.1. PIR Prepolymer
- Polycaprolactone polyol (3.408 g) was weighed and added to silicone surfactant, deionized water, and
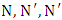 -tris (3-dimethylaminopropyl) hexahydro-s-triazine in a stainless steel cup with continuous stirring for 10 s. 6.3 equivalent amount of 1,6 hexamethylene diisocyanate was added to the content of the stainless steel cup at 80°C and stirred for 6 s.
-tris (3-dimethylaminopropyl) hexahydro-s-triazine in a stainless steel cup with continuous stirring for 10 s. 6.3 equivalent amount of 1,6 hexamethylene diisocyanate was added to the content of the stainless steel cup at 80°C and stirred for 6 s. 2.3.2. PI Prepolymer
- Poly (amic acid) was prepared by weighing pyromellitic dianhydride (PMDA) (1.8 g, 10 mmol), dissolved in DMF at 80°C, cooled to room temperature, and then reacted with 4,4’ oxydianiline (1.652 g, 6 mmol), isoquinoline (3.32g) and benzoic acid (2.93 g, 24 mmol) in a 250 ml threenecked flask equipped with a mechanical stirrer and a condenser. The mixture was heated for 1 h at 70°C and then for 6 h at 190°C. The mixture was cooled to room temperature and precipitated in methanol. The precipitated polyimide prepolymer was collected and dried in a vacuum oven.
2.3.3. Poly(isocyanurate-imide) (PIR-PI)
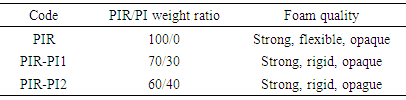
- Poly(isocyanurate-imide) foam was synthesized by weighing PI prepolymer and dissolving in NMP. PIR prepolymer and triphenylphosphine were added to the solution. The blend solution was mixed thoroughly (1500 rpm) for 90s and was immediately transferred to a closed mold. Solvent was evaporated in a vacuum oven at 200°C for 11 h. The foams were removed from the mold and left to cure for 7 days. Different PIR-PI foams were produced by different weight ratios of PIR and PI and were marked by PIR-PI1 and PIR-PI2 (Table 1).
3. Results and Discussion
3.1. FTIR Spectroscopy Analysis
- Polyimide foams with enhanced thermal stability and easily processable were prepared through the reaction of polyimide prepolymers and isocyanate-terminated isocyanurate prepolymers in the presence of a foaming agent. The desired soluble poly (isocyanurate-imide) foams were prepared through a two-step process. Firstly, polyisocyanurate prepolymer was prepared via a one-shot process involving reaction of polycaprolactone, in the presence of an excess amount of HDI to shift the reaction towards creating an isocyanate terminated polymer with functionality for further reactions. Wholly PIR foam was also produced. Figure 1 shows the infrared spectrum of the PIR foam. The FT-IR spectrum showed before the completion of the reaction the presence of isocyanate, indicated at the absorption band 2100 – 2270 cm-1. Other characteristics absorptions are indicated at 3319 cm-1 (N-H stretching, aromatic amine), 3058 cm-1 (C-H stretching, aromatic), 1774 cm-1 (C-H stretching), 1720 cm-1 (C=O stretching, urethane), 1242 cm-1 (C-O stretching).
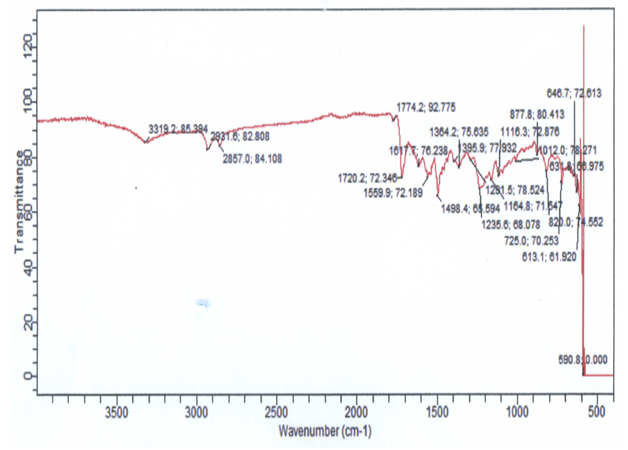 | Figure 1. FTIR of PIR foam |
|
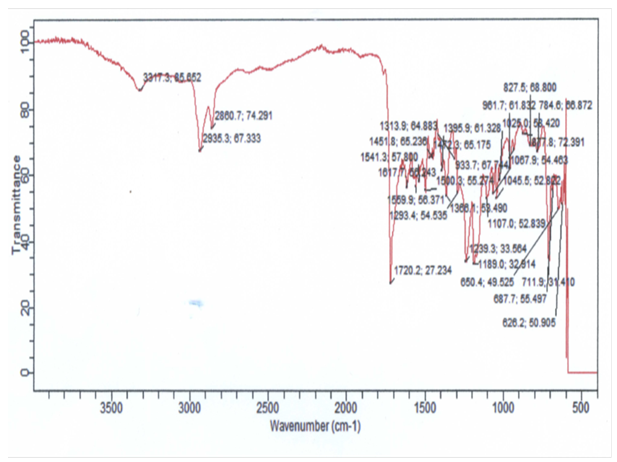 | Figure 2. FTIR of PIR-PI1 |
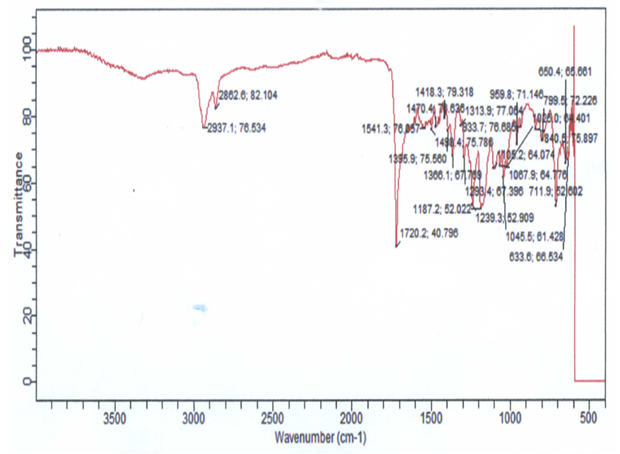 | Figure 3. FTIR of PIR-PI2 |
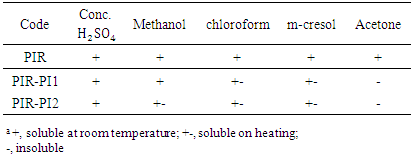
3.2. Solubility Characteristics
- The processability characteristics of the PIR-PI foams are determined by their solubility characteristics (Table 2). Polyimide is generally infusible and insoluble, making it difficult to process. The intent of this study was to introduce flexible pendent structures to the PI backbone chains. Polyimide foam was successfully prepared with isocyanurate without compromise of the strength of the resultant blend. Solubility results showed that blend of polyimide and polyisocyanurate foam particularly PIR-PI1 showed strong solubility in all the solvents, but acetone. Polyimides derived from 70% polyisocyanurate show improved solubility due to the presence of the
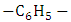 group in addition to the ether group in the chain backbone in comparison with the polyimides derived from 60% polyisocyanurate.
group in addition to the ether group in the chain backbone in comparison with the polyimides derived from 60% polyisocyanurate.
|
3.3. Thermal Properties
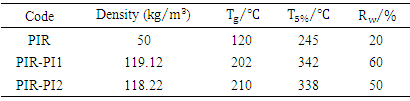
- The thermal properties of PIR-PI foams as well as that of PIR foam were studied using thermo gravimetric analysis techniques. The temperature at which the maximum of loss tangent is observed is commonly defined as the Tg value by TG test. DTG and TGA curves of PIR foam and that of the PIR-PI foams are presented in Figures 4 – 6 and Table 3. From Figures 4 – 6 and Table 3 it can be seen clearly that T5% values of PIR foam and the PIR-PI foams are well above 100°C, the temperature at which decomposition started for all the polymers. The residual weight rates (Rw values) for the PIR-PI foams are more than 40% while that of the wholly PIR foam is 20% indicating PIR-PI foams are more thermally stable at the temperature of measurement, 450°C. The glass transition temperature (Tg) was observed to be in the temperature range 200 – 230°C. The (Tg) decreases with increasing quantity of polyisocyanurate in the polyimide chain. The decomposition behaviors of the PIR-PI foams are similar and two characteristic temperature regions can be observed. In the first region, residual small molecules are volatilized and unstable urea groups are decomposed in the ranges from 250°C to 350°C, while in the second region ranging from 362.5°C to 511.4°C, there was decomposition of the imide ring.
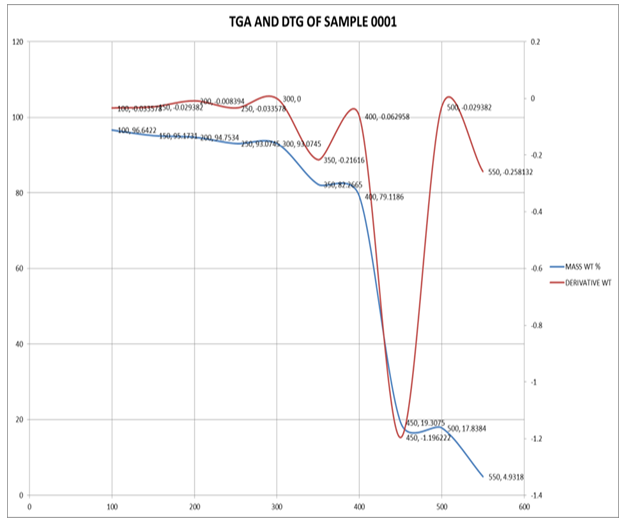 | Figure 4. TGA and DTG characteristics of PIR |
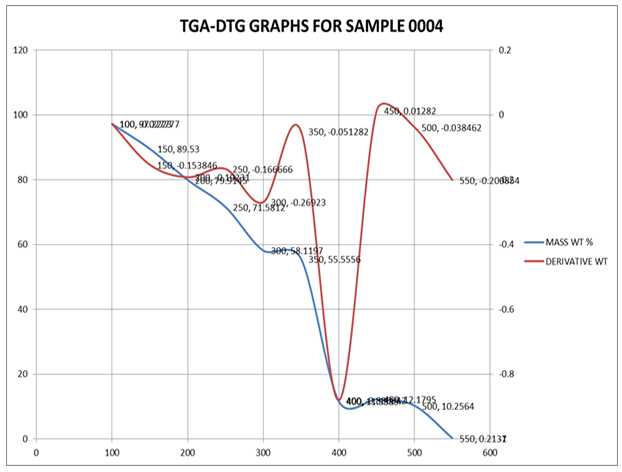 | Figure 5. TGA and DTG characteristics of PIR-PI1 |
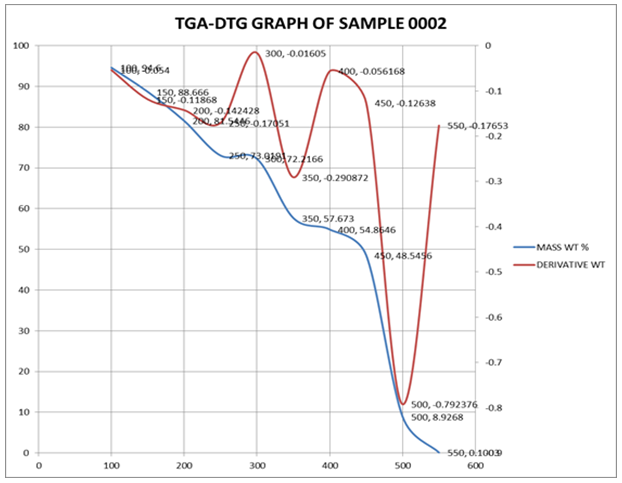 | Figure 6. TGA and DTG characteristics of PIR-PI2 |
|
4. Conclusions
- PIR-PI foams have been prepared by the reaction of PI and PIR prepolymers with 30/70 and 40/60 weight ratios of the prepolymers in the presence of suitable catalysts and deionized water as the foaming agent. Easy of processability as determined by solubility and resultant thermal stabilities of the modified PIs were determined. The result showed that the PIR-PI foams were soluble in chloroform on heating, soluble in H2SO4 at room temperature and insoluble in acetone. Where solubility occurred, PIR-PI foam containing 70% polyisocyanurate showed significantly improved solubility. Using TGA and DTG tests thermal stabilities of PIR-PI foams were studied and the results showed that modification of PI with PIR have substantial effect on thermal stability. From the study, it can be seen that PIR-PI foam can be considered in application for high temperature as promising processable polymeric materials.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML