-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Polymer Science
p-ISSN: 2163-1344 e-ISSN: 2163-1352
2017; 7(2): 38-43
doi:10.5923/j.ajps.20170702.03

Polymeric Compositions on the Base of Acrylic Acid and Bentonite Clay
M. A. Mahkamov, M. G. Muhkamediev
Department of Chemistry, National University of Uzbekistan, Tashkent, Uzbekistan
Correspondence to: M. A. Mahkamov, Department of Chemistry, National University of Uzbekistan, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In this investigations some peculiarities of obtaining composition hydrogels (CH) on the base of cross-linked polyacrylic acid (PAAc) and bentonite clay (BC) were investigated. Optical microscopy and X ray diffraction have shown that in CH’s destruction of crystalline structure of montmorillonite has carried out owing to penetrative of polymeric macromolecules between bundle layers in result of which CHs have a uniform homogeneous structure. The kinetics of swelling of obtained CH in water was investigated and it was shown that they have a high sorption ability to water in wide interval of pH. Sorption of methylene blue (MB) by gels from water solutions was investigated by statistical method. It was determined that sorption ability of CHs was higher than by hydrogels on the base of PAAc. Sorption of MB has increased with increasing temperature of medium and consequently the bonding of MB by CHs has carried out owing to chemisorption.
Keywords: Acrylic acid, Bentonite, Composition hydrogel, Swelling, Sorption
Cite this paper: M. A. Mahkamov, M. G. Muhkamediev, Polymeric Compositions on the Base of Acrylic Acid and Bentonite Clay, American Journal of Polymer Science, Vol. 7 No. 2, 2017, pp. 38-43. doi: 10.5923/j.ajps.20170702.03.
Article Outline
1. Introduction
- Polymeric hydrogels (PH) are objects of practical interest owing to possibilities of their using in many fields of science and industry for solutions of different problems. PHs can be used in medicine as medical compounds; for division and adsorption of ions of different metals; in ecology for purification of sewage from different organic and inorganic impurities; for obtaining sensors [1-7]. The base of PHs in most cases is synthetic polymers, distraction of which in natural conditions is difficult what is their lack from the point of ecology. By this reason construction of absorbents on the base of biodegradated materials and decreasing in their composition the content of synthetic polymers is one of the important task. The obtaining of compositions on the base of cross-linking polymers by introductions in their composition of different natural materials is one of possible ways of decision of this problem. By this reason polymer-bentonite compositions have the special interest. The choice of bentonite clay (BC) as component for polymeric compositions is determined by its high hydrophilicity, low toxicity, ecological safety; the good adsorption ability and also that it is accessible and cheap material. These properties of BC’s have allowed to consider it as the most perspective material for obtaining composites. Authors [8, 9] have shown that BC particles have attached to gels some new phisico-chemical properties and also improved their mechanical properties. Therefore construction of composition hydrogels (CH) on the base of such system as polymer-BC and investigation their phisico-chemical properties have allowed to decide some problems arising at PHs using.The aim of this work is synthesis of CHs on the base PAAc gel with inclusion of BC particles and investigation of some physico-chemical properties of obtained materials. According to aim, PHs were obtained on the base of PAAc and CHs by radical polymerization of acrylic acid and cross-linking agent adsorbed on the BC. Investigation of the sorption degree of obtained materials was carried out. Sorption of methylene blue (MB) by synthesized materials from water solution in statistical condition was investigated for determination of their sorption ability.
2. Experimental
2.1. Chemicals
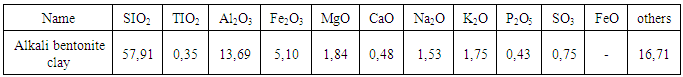
- Acrylic acid (AAc; OAO Reactive, Russia) was vacuum distilled at 47°C/ 7mm Hg. The cross-linker N,N’-methylene-bisacrylamide (N,N’-MBAA; BDH Chemical Ltd, England) was of analytical grade. Methylene blue or methylthionimium chloride (MB, Russia) is a dye having also antiseptical properties. Bentonite clay (Uzbekistan) of grade “Navbahor” has following composition, mass %:
 and it before using was washed and dried on the air to constant mass and then was carefully reduced to fragments.
and it before using was washed and dried on the air to constant mass and then was carefully reduced to fragments.2.2. Synthesis of Hydrogels and Composition Materials
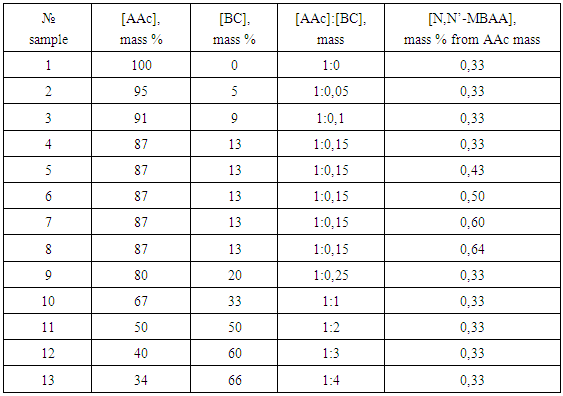
- PHs were obtained by polymerization of acrylic acid (AAc) in water solution in the presence of cross-linking agent N,N’-MBAA. Oxidized-redoxy system on the base of thiosulfate sodium and persulfate potassium was used as initiator. Reaction was carried on at 25°C during 24 h. The obtained gels were placed in glass column and were washed by distillated water during 20 h and then were dried at temperature 45°C to constant mass.For obtain of CH firstly the suspension of BC in water at mixing by magnet stirrer during 2 h was prepared. Than to this suspension the monomer and cross-linking agent (AAc+N,N’-MBAA) were added and the obtained system was mixing during 6 h. Than to suspension the oxidized-redoxy system was added and mixture was poured in test-tubes. The copolymerization was carried out during 20 h at 25°C. After copolymerization the obtained gels were extracted from test-tubes, then they were purified from residues of monomers by repeated washing in column by distillated water and were dried at temperature 45°C to constant mass. Conditions of obtain of some investigated systems are presented in Table 1.
|
2.3. Investigations of Gels Rheology
- Roentgenograms of gels samples and compositions were recorded on the DRON-3 (Russia) at wave length 1,54 A. Microphotographies of PHs and CHs samples were recorded on the optical microscope BIOLAM-6 (Russia).
2.4. Degree of Swelling
- Swelling degree of PHs and CHs in water was determined by the gravimetric method in special cells supplied by net from nylon polymeric material. The values of the swelling degree of hydrogels (Q) were calculated by following formula:
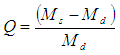 were: Ms and Md – the masses of swelling and dry samples.
were: Ms and Md – the masses of swelling and dry samples.2.5. Sorption of Methylene Blue by Gels
- Sorption of MB from water solutions of PHs and CHs was investigated by the spectrophotometric method. Samples of gels with equal mass were placed in water solutions of MB and through some intervals of time the optical density of solutions (D) was measured on the SF-46 (Russia) at wave length 500 nm. MB concentration in solutions was determined on the base of calibrated graphic in coordinates: concentration of MB in solution- optical density.
3. Results and Discussion
3.1. Obtain of Hydrogels and Investigation Their Rheological Parameters
- It is known that bentonits are an minerals with high content of montmorillonite which has formed very small leafs and fiber like isolations. Investigation with using of electronic microscope has shown the presence in it some characteristical lamellar, petal-like crystals. Crystalline lattice of montmorillonite has an ability to expansion [11, 12], what is caused by it’s atomic structure. If inside of lamellar bundles in montmorillonite there are covalent bonds then between bundles there are only weak strengths of Van-der-Vaals. By this reason water and other polar liquids can easily penetrate in space between the bundles and the expansion or swelling of montmorillonit lattice has carried out. This can caused to increasing of distance between bundles in several times. High changing capacity of the montmorillonite in comparison with some other clay minerals also can be explain by fact that in it’s crystals the ionic exchange has carried out not only on the outer surface of crystals but also inside of the crystalline lattice between atomic layers. Correspondently at water sorption the swelling was carried out not only owing to formation of solvatic covers on the surface of bundles but also to introduction of water molecules between bundles of the crystalline lattice [13].It is obvious in this case that at addition of monomers in BC suspensions their penetration can carried out between bundles layers of crystalline lattice of montmorillonite (Fig. 1.b). At polymerization of some monomers the nettings are formed the cells of which are filled by BC crystals (Fig. 1.c). Such scheme of formation of the polymeric composition was presented in work [14].
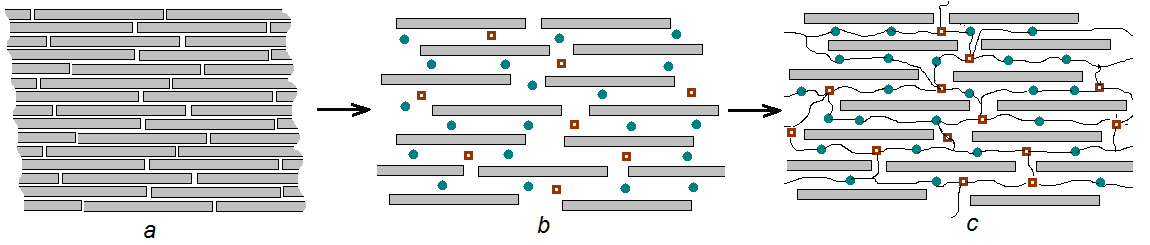 | Figure 1. Scheme of the compositions hydrogels formation on the base of cross-linking PAAc and BC |
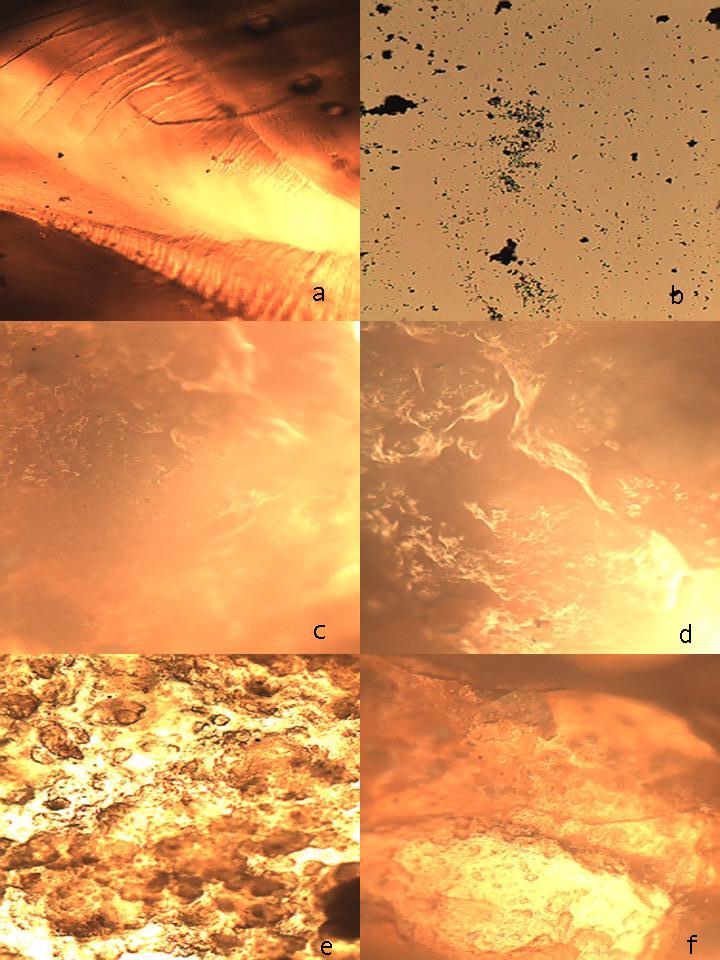 | Figure 2. Microphotographies of the cross-linking gel on the base of PAAc (a); particles of BC (b) and CH (content of BC in composition: c-33; d-50; e -60 and f-66 mass %) |
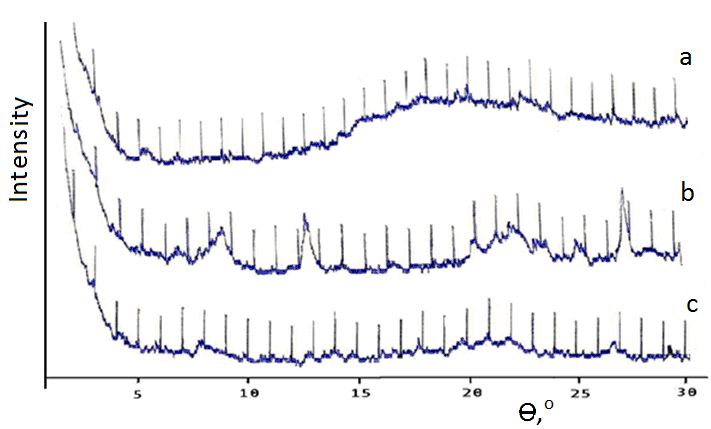 | Figure 3. Diffraction patterns of gel samples on the base of PAAc (a), BC (b) and CH (c). Content BC in CH - 33 mass %) |
3.2. Swelling of Obtained Materials
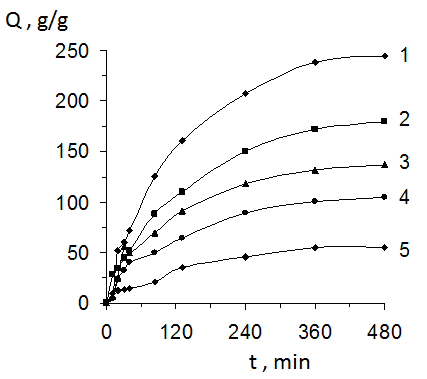
- One of the important parameter of hydrophilic gels is their degree of swelling an by this reason kinetics of swelling of obtained materials in water solutions was investigated. In the case of CHs it was shown that the equilibrium at swelling of complexes has been achieved during 6-7 h (Fig. 4). The increasing of content N,N’-MBAA in gels has carried out to decreasing their swelling degree.
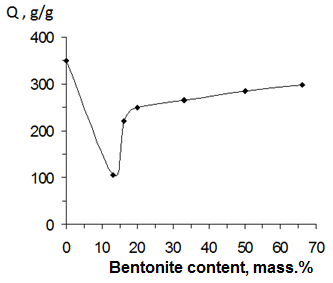 | Figure 5. Dependence of the degree of equilibrium swelling of CHs from content BC in their composition (temperature 25°C) |
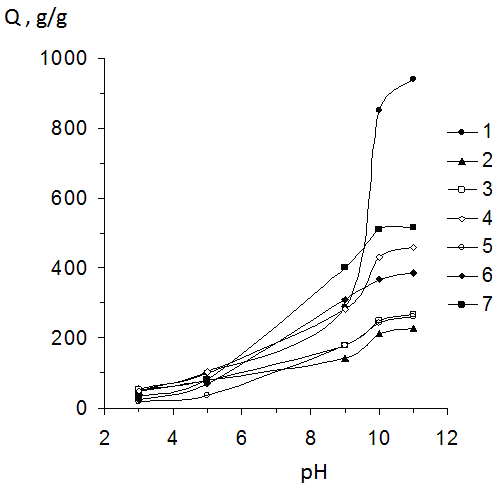 | Figure 6. Influence of pH solution on the swelling degree of PHs and CHs. 1,2,3,4,5,6,7-content of BC in CHs correspondently equaled 0; 9; 13; 20; 50; 60 and 66 mass %. (temperature 25°C) |
3.3. Sorption
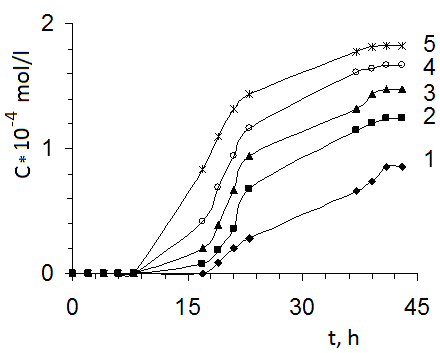
- One of the important characteristics of PHs is their sorption ability in relation to different organic and inorganic compounds. By this reason absorption of MB from water solutions by obtained gels was investigated. Sorption of MB by hydrogels on the base of PAAc and CHs from water solutions was investigated in staticall conditions. Kinetics of MB sorption by polymeric material is presented on Fig. 7.
|
4. Conclusions
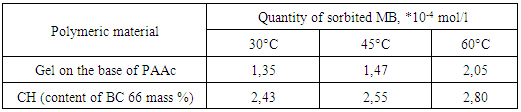
- Thus in this work CHs on the base PAAc and BC were obtained. It was shown that obtained materials have an homogeneous structure. Investigation of the swelling degree of CHs in water has shown that this parameter has depended on BC content. With increasing of BC content in gels their swelling degree firstly decreased and than constantly increased. Investigation of pH influence on the equilibrium degree of swelling had shown that with increasing of BC content in gels their sensitive to pH changing decreased. Sorption of MB by obtained sorbents was investigated and also it was shown that CHs possessed more sorption capacity in comparison with gels on the base of PAAc. It was determined that sorption of MB has beginned after swelling of composition materials. The increasing of temperature has resulted in increasing of MB sorption which had a chemical character. Thus the possibility of obtain of effective absorbents on the base of composites PAAc-BC has been shown.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML