-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Polymer Science
p-ISSN: 2163-1344 e-ISSN: 2163-1352
2017; 7(2): 23-29
doi:10.5923/j.ajps.20170702.01

Influence of Gamma Irradiation on Structure and Properties of Nitrile-Butadien Rubber in Presence of Modified Nano Metals
Mammadov S. M.1, Khankishiyeva R. F.1, Ramazanov M. A.2, Akbarov O. H.2, Akbarov E. O.2, Akhundzada H. N.1
1Institute of Radiation Problems of ANAS, Azerbaijan
2Baku State University, Azerbaijan
Correspondence to: Mammadov S. M., Institute of Radiation Problems of ANAS, Azerbaijan.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Polymer-based nanocomposites NBR in the presence of nanoscale zinc oxide powder were obtained the crosslinking agent disulfochlorid benzene (DSCB) and carbon black. The role of ZnO nanopowders and DSCB in NBR systems NBR+ZnO+DSCB and NBR+ZnO+DSCB+F324 with radiation, chemical exposure. With the help of physico-chemical and spectral methods shown changes in the molecular structure of the rubber NBR, in the presence of ZnO nanopowders and DSCB. Each studied nanocomposites have been identified by and the emergence of cross-linking and cross-linking yield in the rubber. It is shown that the presence of the chemical interaction between polar groups of the macromolecule BNK (-C≡N) DSCB (C-Cl) nano particles of zinc oxide leads to a significant change in the density of spatial grid nanoexamples that provides an increase in the output of cross-links (1/Ms). Shown properties of filled nanocomposites prepared by chemical bonds (C-C) to modify the mechanical properties in the 423K and 500kGy. Especially, conductive properties of nanocomposites are identified based on NBR. It is found that the change in the value of dielectric permittivity (ε) and dielectric losses (tgδ) material depends on chemical reactions of the polymer structure.
Keywords: Butadiene nitrile rubber, Nanocomposite, Vulcanisation, Gamma irradiation, Disulfochloridebenzene
Cite this paper: Mammadov S. M., Khankishiyeva R. F., Ramazanov M. A., Akbarov O. H., Akbarov E. O., Akhundzada H. N., Influence of Gamma Irradiation on Structure and Properties of Nitrile-Butadien Rubber in Presence of Modified Nano Metals, American Journal of Polymer Science, Vol. 7 No. 2, 2017, pp. 23-29. doi: 10.5923/j.ajps.20170702.01.
Article Outline
1. Introduction
- An effective technique for the improving the technical parameters and the spatial grid of polymer systems is the introduction of these nanoscale inorganic modifiers. [1, 2] The action of these modifiers is often due to their influence on the process crosslinking of polymers, including the ability to form a chemical bond [3-5]. In the last decade, actively carried out the study of polymeric nanoparticles of metals, semiconductors [6-8], which is together good processability, have improved wear resistance, high hardness and elasticity. The highly developed surface, as well as the proximity of the nanoparticle size to the size of the macromolecules lead to forming nanocomposites unique electronic, electro-mechanical and physical properties are very promising for the creation of new constructional materials [9, 10] used for new equipment, solid and elastic defense materials. Significantly, the desired effect is achieved by introducing a small amount of modifier.One of the attractive type nano modifiers are oxides of metals (OM) [11] which are several levels were equally dispensed in the polymer type matrix. [12]It is believed that the reason for the unique effect of OM polymer systems is their large chemical activity associated with nanoparticle size (2-100nm).Thus, OM can have a double effect: to act as structures for the adjustable matrix or the ability of the formation of additional nodes, mesh links in elastomeric systems.Initiating crosslinking with OM and the possibility of reproducing the particles in the polymer matrix may also determine the improvement in mechanical properties of nanocomposites. Thus, the amplification effect: increase in dynamic endurance and tensile strength was observed with the introduction of the OM in the ethylene-propylene copolymer. [13]Recent advances in the field of processing of nitrile rubber (NBR) have led to the expansion of their modifications and allowed to receive new crosslinked elastomeric materials. The completed earlier studies of the structure and mechanical properties of various compositions NBR, showed that the NBR has a complicated structure, including C-C and C-S-C bond. [14]Such a set of cross-linking provides large opportunities for the necessary complex by their structural adjustment properties. Several works shown that, introduction to active, crosslinked and reactive low molecular weight compounds (LWC) [15] results in a substantial improved in mechanical properties. However, these studies are quite limited and do not provide a complete picture of the mechanism of the observed effects and the conditions of manifestation. NBR differ significantly greater tendency to cross-linking radiation, other than diene elastomers. Since it is cured valuable technical material can be obtained even when irradiated without additives.Radiation-chemical synthesis of NBR in the homogeneous crosslinking, as a rule, complicated by the reactions of the polymer chains. [16] Due to the fact that the unsaturated elastomer is NBR based on the ability of the polymer molecules in principle the radiation crosslinking is carried out using a fairly wide range of substances. NBR is a convenient object for studying the impact of nano-sized powders OM, and DSCB crosslinking agent that is active polar (Cl) and an aromatic group, which allows you to assess the impact of these groups on the parameter space grid and thermal radiation vulcanizates.This work aims to address these problems.
2. Methodology and Research Facility
- In this work, for nanocomposites using a high-molecularly nitrile butadien rubber (NBR) and the content of acrylic nitrile (AN) was 40% in the molecule. NBR is obtained by co-coagulation of nitrile butadiene latex in the ratio of 60:40. As a result, studies by the Fourier method of IR spectroscopy in the butadiene portion of the polymer composition of the isomeric double bonds in the elastomer investigated 1,4-isomer and 14.8% trans-1,4 isomers 70.9%.Macromolecules rubber NBR was distributed from the static of butadiene and acrylonitrile.Features crosslinking NBR affect their microstructure, molecular weight distribution (MWD) and gel content. Therefore, prior to the radiation-chemical processes in the elastomer pre osmometric method and penetrating gel chromatography (PGC), determined by the average number and molecular weight and polydispersity (Мn=69 thousand, Мw=226, Мn:Мw=4,1).As activation crosslinking polymer used nano zinc oxide (ZnO). With the introduction of ZnO nanopowders pay attention to the dimension (20-25nm) dispersion and purity (99.8). Specific surface (250 g/m2), true density 5.606 g / sm3. Powder dosage was varied in the range 0.8-4.0 phr. The nanomaterials had been obtained from the Inc. Houston, TX, USA.In the process of further agent (DSCB) aromatic compounds, which react readily with macromolecules NBR. DSCB is an organosulfur compound with the formula C6H5(SO2)2Cl2 It is a colourless viscous oil that dissolves in organic solvents, but reacts with compounds containing reactive N-H and O-H bonds.The effect of technical carbon phase was judged by the change in the rheological and mechanical properties.With the introduction of fillers in polymer systems we have noticed a chemical reaction at the interface of the two phases, adsorption, dispersion, specific adsorption and surface geometry (78-90, 80-88 m2/g) and the impact of carbon black furnace (F324) on the formation of carbon rubber gel (CRG) in the elastomer. Nanocomposites based on NBR which are prepared by the laboratory rollers with a fraction f = 1: 2. Firstly added rubber (100 phr), Then added nanopowder 5.0 phr ZnO, then at the end 3,0 phr DSCB. For filled mixture is introduced at the end of 50 wt. h. P324 filler (carbon black) mixing on rollers constituted to binary (1) and quasi systems (2, 3) 12-15 min. 1. NBR + ZnO;2. NBR + ZnO + DSCB; 3. NBR + ZnO + DSCB + F324Studies were carried out on films and plates (60x60 mm) obtained by compression of mixtures at 423 K, followed by cooling.For the irradiation of the samples were placed in a glass (1 g) and vacuumed for hour, under the 1,3х10-1 Pa residual pressure. All the samples were irradiated under the Co-60 gamma-ray source at absorbed dose of 4.9 kGy at room temperature.Absorbed dose of samples calculated by comparing the electron densities in the dosimetry systems. [17, 18]Studies were carried out on films and plates (60x60 mm) obtained by compression of mixtures at 423K, followed by cooling.The molecular structure and isomeric composition of nanocomposites NBR was studied by Fourier Transform Infrared Spectroscopy (FTIR) in the 700-3000 cm-1 rubber films were prepared by applying the solution (toluene) to a substrate and evaporation of the solvent constant.The intrinsic viscosity (IV) and quasi nanobinaric systems determined in toluene at 293 K in a known manner Ubbelohde viscometer. The calculation was performed by the Mark Houwink [ŋ]=KMα value of constant К=1,9×104 and α=0,64 for toluene. [19]Effect of OM on the parameters of the output concentration of the number of grid lines (1/Ms) was determined by the sol-gel analysis. Calculation of the spatial grid of cross-linked polymer parameters determined by the sol-gel analysis and by the Flory–Rehner equation. [20]The mechanical properties of the vulcanizates were determined by a universal testing machine (UTM), in accordance with ASTM D412, displaying results supports communication via LAN and Internet.Measurements of the electrical properties (ε', tgδ) of nanocomposites was performed using the bridge P 5083 in the frequency range of 102-105 Hs, which has been linked with the measuring cell clamping electrodes [21]. Samples were prepared for investigation in the form of films with a thickness of 140-160 microns and a diameter of 25 mm by a pressing operation at T>Tc and P=107 Pa, followed by cooling under pressure to room temperature at a rate of 276 Grad / min.
3. Results and Discussion
- Fourier spectroscopy methods describe that the ZnO particles is well distributed in the matrix of NBR, the system NBR-ZnO-DSCB characterized polarity so the process of combining NBR nano zinc oxide with DSCB mainly determined -C≡N polar groups and C-Cl their reactivity under thermal and radiation effects.On this basis, the main attention was paid to the intensity of the band stretching vibration of the nitrile group, located in the area of 2235 cm-1 (Fig. 1) and are characterized by exceptional stability, both in frequency and form.
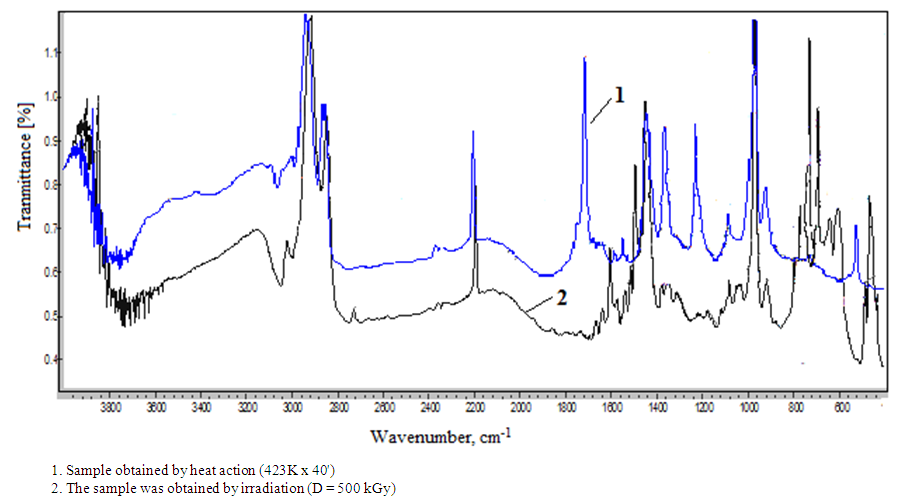 | Figure 1. Fourier transform infrared spectroscopy (FTIR) system of nanocomposite filled thermal and irradiated nanosamples (NBR + DSCB + ZnO) |
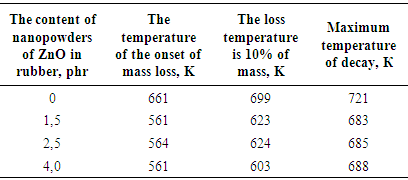
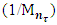 decreases due to consumption of double bonds, but the formation of cross-links in one sm3 vulcanizate.
decreases due to consumption of double bonds, but the formation of cross-links in one sm3 vulcanizate.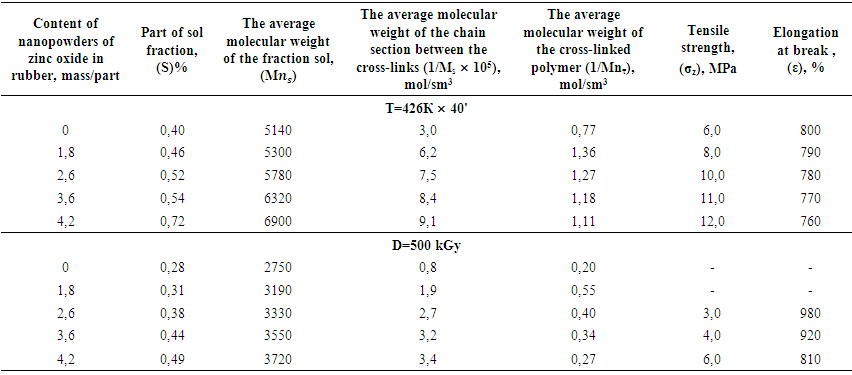 | Table 1. The basic properties and structural parameters of the thermal (426K x 40') and radiation (D = 500 kGy) samples of activated zinc oxide nanopowders |
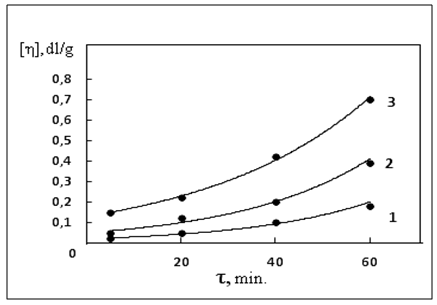 | Figure 2. The kinetics of change of the intrinsic viscosity polymer systems of warm-up duration: 1. NBR; 2. NBR-ZnO; 3. BNK-ZnO-DSCB |
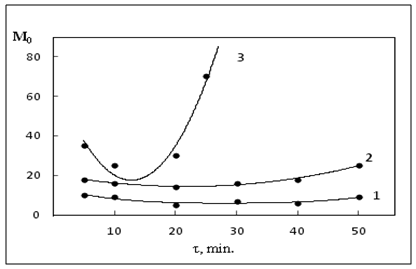 | Figure 3. Kinetics of change in viscosity nanocomposites NBR-DSCB and DSCB+NBR+ZnO in the time depended crosslinking (423K x 40') 1. NBR; 2. NBR-DSCB; 3. NBR-DSCB+ZnO |
|
|
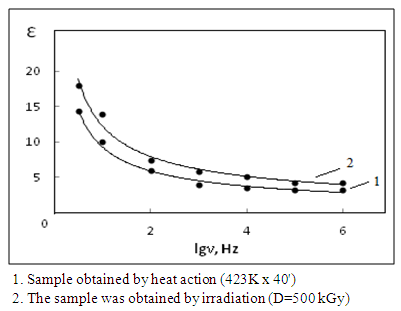 | Figure 4. Frequency dependences of the changes in the permittivity of filled nanocomposites (NBR + DSCB + ZnO + F324) |
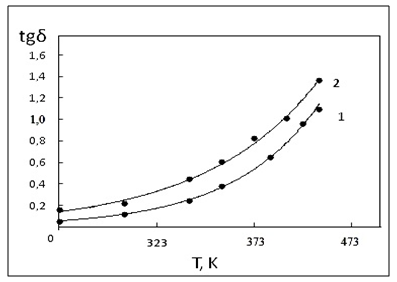 | Figure 5. Temperature dependence of dielectric losses filled thermal |
4. Conclusions
- Thus, polymeric nano composite materials were prepared in the presence of an additive of a nanoscale zinc oxide powder containing a crosslinker of DSCB and carbon black. Introduction of nanopowders zinc oxides causes an acceleration of the process of NBR crosslinking. The highest binding speed of nanocomposites and the greatest number of cross-links in the mixture is achieved by the introduction of DSCB.In all probablities, their ability to polarize the double bond of both the polymer and the cross-linking agent, which leads to the formation of metal-containing polymeric nanocomposites characterized by a uniform, narrow dispersed distribution of ZnO nanoparticles 20-25 nm in the polymer matrix. At the same time, it should be noted that the activating ability of the ZnO nanopowder is influenced by the specific surface area.The data obtained indicate that when the nanopowder ZnO is used together with technical carbon in the polymer, the mechanical properties of the nanocomposites. The reason for the increased strength can be as a large density of the polymer mesh, and the presence of transverse bonds of adsorption nature.These effects of gamma irradiation on the nature of the radiation-chemical yield of crosslinking in nanocomposites are not large compared to thermal crosslinking.The carried out researches allow to assert, that change of size of dielectric constant (ε) and dielectric loss (tgδ) depends on a density of a grid of nanocomposites of a specific surface of technical carbon, ZnO and an irradiation dose.
ACKNOWLEDGMENTS
- This work supported by the Science Development Foundation under the President of the Republic of Azerbaijan-Grant № EIF-KETPL-2-2015-1(25)-56/10/1
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML