-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Polymer Science
p-ISSN: 2163-1344 e-ISSN: 2163-1352
2017; 7(1): 15-22
doi:10.5923/j.ajps.20170701.03

Comparative Physicochemical Characterization of Chitosan from Shells of Two Bivalved Mollusks from Two Different Continents
Stephen O. Majekodunmi, Emmanuel O. Olorunsola, Cynthia C. Uzoaganobi
Department of Pharmaceutics and Pharmaceutical Technology, University of Uyo, Uyo, Nigeria
Correspondence to: Stephen O. Majekodunmi, Department of Pharmaceutics and Pharmaceutical Technology, University of Uyo, Uyo, Nigeria.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The need to turn seafood wastes that hitherto constitute an environmental pollution into important pharmaceutical excipients is gaining ground worldwide. This study investigated the extraction and characterization of chitosan from two oyster shells: Mytilus edulis and Laevicardium attenuatum from different continents. Demineralization and deproteinization were carried out to obtain chitin from the shells, followed by deacetylation to obtain chitosan. Percent yield, degree of deacetylation and other physicochemical characteristics were determined for the extracted chitosan from the two oyster shells. The yield of chitosan from Mytilus edulis was 51.8% and 43.8% from Laevicardium attenuatum. The degree of deacetylation (DD) of chitosan from Mytilus edulis was 69.6% while that of Laevicardium attenuatum was 37.3%. The Laevicardium attenuatumchitosan had higher swelling ratio. Calcium was the predominant metal ion in the two chitosan. While the micrograph of Mytilus edulis chitosan showed a non-uniform particle distribution, Laevicardium attenuatum chitosan showed a brick-like structure. This work has shown that it is more economical to produce chitosan from Mytilus edulis, and the polymer has higher degree of deacetylation. The higher degree of deacetylation implies better solubility and the chitosan could be more suitable as a permeation enhancer. The Laevicardium attenuatumchitosan could be more suitable for sustained release formulation.
Keywords: Chitosan, Mytilus edulis, Laevicardium attenuatum, Oyster shells
Cite this paper: Stephen O. Majekodunmi, Emmanuel O. Olorunsola, Cynthia C. Uzoaganobi, Comparative Physicochemical Characterization of Chitosan from Shells of Two Bivalved Mollusks from Two Different Continents, American Journal of Polymer Science, Vol. 7 No. 1, 2017, pp. 15-22. doi: 10.5923/j.ajps.20170701.03.
Article Outline
1. Introduction
- Economic hardship globally calls for turning environmental waste products into useful pharmaceutical excipients such as chitin and chitosan. Chitosan is a polymer derived from deacetylation of chitin. Chitin is naturally obtained from several sources which include cell wall of fungi, exoskeleton of crustacean such as crabs and shrimp; insects, oyster shells and other marine animals [1]. Oyster shells are seen littering beaches, river banks and markets (after the animals have been removed and used as food). Attempts to burn them further constitute more environmental nuisance [2].Chitosan is a linear polysaccharide composed of randomly distributed β-(1-4)-linked D-glucosamine and N-acetyl-D-glucosamine. It is produced by treating the shells of the marine animals with the alkali sodium hydroxide. The amino group in chitosan protonates in acidic to neutral solutions making the polymer to be water-soluble at this pH range. It is a bioadhesive capable of binding to negatively charged surfaces such as mucosa membranes [3, 4]. Chitosan is biocompartible and biodegradable and it enhances the transport of polar drugs across epithelial surfaces. Therefore, chitosan has a number of commercial and biomedical uses. In medicine, it may be useful in bandages to reduce bleeding and as an antibacterial agent. It is also useful as a soluble dietary fiber.In pharmaceutical industry, chitosan is useful in sustained delivery of drugs. It has also been employed in oral drug formulations in order to improve the dissolution of poorly soluble drugs [5]. Chitosan microspheres have also been produced for use in enhanced chromatographic separation [6], for the topical delivery of drugs [7], for drug targeting after injection [8] and for controlled release of drugs [9-11].Mytilus edulis (Phylum mollusca, class bivalvia, Family Mytilidae) commonly called mussel, is an oyster animal which live on exposed shores in the intertidal zone (Figure 1). In most marine mussels, the shell is longer than it is wide, being wedge-shaped or asymmetrical. The external color of the shell is often dark blue, blackish, or brown, while the interior is silvery and somewhat nacreous. The word “mussel” is most frequently used to mean the edible bivalves of the marine family. Freshwater mussel species inhabit lakes, ponds, rivers, creeks, canals and they are classified in a different subclass of bivalves, despite some very superficial similarities in appearance. The shells of mussel are found scattered all over Long beach in the United States of America.
 | Figure 1. Photograph of Mytilus edulis |
 | Figure 2. Photograph of Laevicardium attenuatum |
2. Materials and Methods
2.1. Materials
- Shells of Mytilus edulis were obtained from Long beach, California, USA where they were seen scattered all over the beach. Laevicardium attenuatum shells were picked on Lagos beach, Nigeria. Other materials used were sodium hydroxide pellets, potassium permanganate crystals and 36% v/v hydrochloric acid (all from BDH Chemicals, England). Distilled water was prepared in the Process Laboratory of Department of Pharmaceutics and Pharmaceutical Technology, University of Uyo, Uyo, Nigeria. All other reagents used are of analytical grade.
2.2. Extraction of Chitosan
- The two shells were washed separately and oven dried for four days. They were blended and then sieved to obtain 250 microns powder size. The 250 microns powder size was subjected to serial extraction. Extraction was carried out firstly by deproteination, followed by demineralization and then deacetylation processes using the method earlier reported by [2].
2.3. Determination of Yield of Chitosan
- The percentage yield was calculated using equation 1.
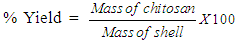 | (1) |
2.4. Determination of Degree of Deacetylaton (DD)
- The degree of acetylation was determined using acid – base titration method with modification as described by [15].
2.5. Determination of Moisture Content
- A 2 g sample of polymer was weighed and transferred into an electronic moisture analyzer (Type MB 35, OHAUS, Switzerland) and the percent moisture content was determined.
2.6. Determination of Viscosity
- The viscosity of 2% w/v dispersion of each polymer was determined using a DV1 prime viscometer (Brookfield Engineering, U.S.A.). About 80 ml of the polymer dispersion was placed inside the cup of the viscometer and the reading was taken at 60 rpm (spindle No.1).
2.7. Determination of Swelling Ratio
- A 2 g sample of the polymer was placed inside a 10 ml measuring cylinder. The tapped volume was taken and the cylinder was filled with distilled water to the 10 ml mark. The mixture was mixed thoroughly and then allowed to stand for 6 h after which the swollen volume of the polymer was taken. The swelling ratio was calculated as the ratio of the volume of the swollen mass to the tapped volume.
2.8. Fourier Transform Infrared (FTIR) Spectroscopy
- Infrared spectrophotometer (Shimadzu IR Prestige 21, China) was used in analyzing the sample of chitosan from the two different shells in the range of 400 to 4000cm-1.
2.9. Scanning Electron Microscopy (SEM)
- The structures of chitosan from the two shells were examined using electron microscope (Model SEM PROX, Phenomworld, Eindhoven, Netherlands).
2.10. X-ray Diffraction (XRD)
- The degree of crystallinity was detected by XRD analysis at room temperature by the use of materials analyzer diffractometer (Model: EMMA, GBC Scientific Equipment Pty Ltd., Australia) equipped with Cu target X-ray tube with wavelength of 1.54059 Å and step size of 0.05. X-ray diffraction was taken in the 2θ range of 5° to 70°. The mineral search was carried out using the GBC X-ray analysis TRACES Software (Version 6) which operates on the full ICCD PDF-2 database file.
2.11. Energy Dispersive X-ray Fluorescence (XRF)
- X-ray fluorescence (XRF) spectrometer (Model: EDX3600B, Skyray instrument Inc., USA) was used to determine the quantitative and qualitative elemental analysis of chitosan extracted from shells of Mytilis edulis and Laevicardium attenuatum. XRF detects elements between sodium (Na, Z = 11) and Uranium (U, Z = 92) with high resolution and fast analysis.
2.12. Statistical Analysis
- Experimental data have been represented as the mean with standard deviation (SD) of different independent determinations. The significance of differences was evaluated by analysis of variance (ANOVA). Differences were considered statistically significant at p < 0.05.
3. Results and Discussion
3.1. Some Physicochemical Properties
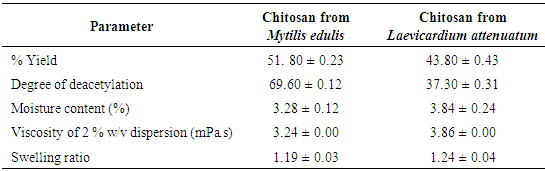
- The results of the % yield, degree of deacetylation, moisture content, viscosity and swelling ratio of chitosan from Mytilus edulis and Laevicardium attenuatum are shown in Table 1. The yield varied from one animal to another within the same class. The higher yield from Mytilus edulis could be due to effective demineralization and deproteination steps. The lower yield from Laevicardium attenuatum may be as a result of the time length of the deacetylation process resulting in depolymerization of the chitosan polymer that could have led to loss of material from excessive removal of acetyl groups from the polymer during washing [16]. Divya et al., [17] extracted chitosan from crab shell and reported yield in the range 30-36.7%. They suggested that the difference in yield was due to the reaction time as well. Puvvada et al. [18] reported a yield of 34% which is lower than those of the two experimental shells.
|
3.2. FT-IR Spectra of Chitosan from the Two Sources
- The FTIR spectra of chitosan from Mytilus edulis and Laevicardium attenuatum are shown in Fig. 3 and Fig. 4 respectively.
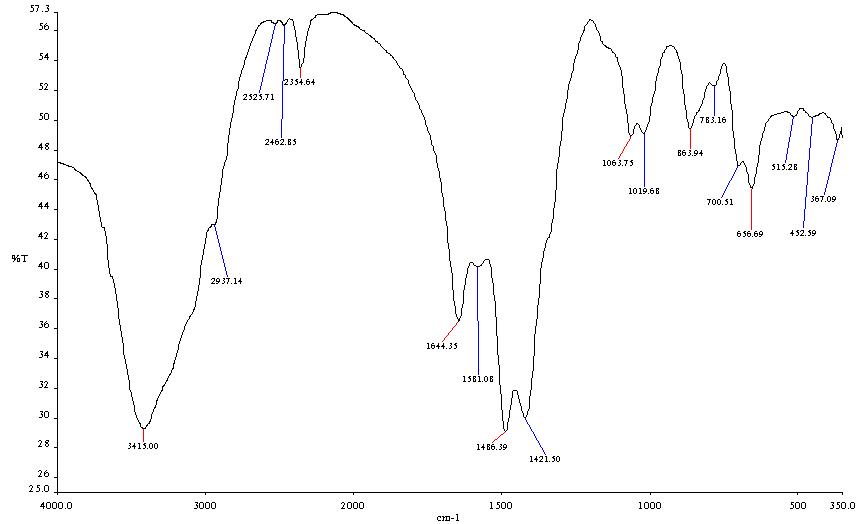 | Figure 3. FTIR Spectrum of Mytilis edulis |
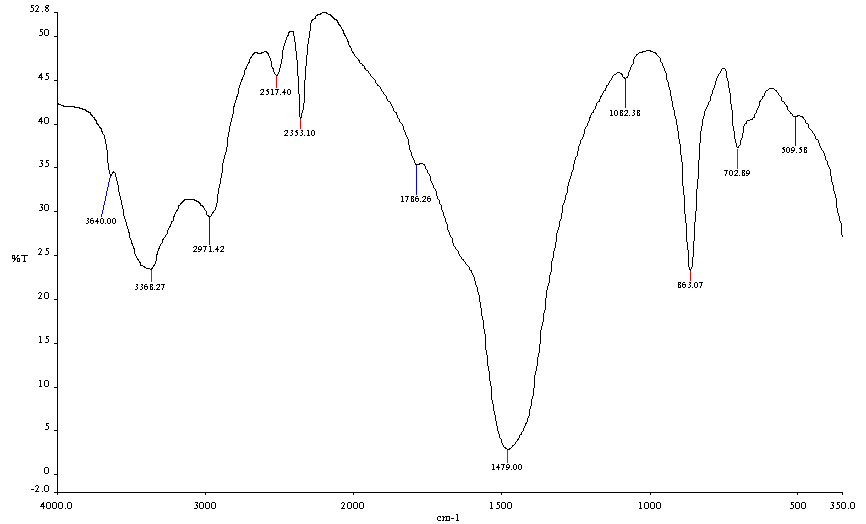 | Figure 4. FT-IR Spectrum of Laevicardium attenuatum |
3.3. Scanning Electron Microscope Analysis
- The surface morphology of the two shell samples is shown in Fig 5. The particle surface of Mytilus edulis chitosan became rougher as the magnification was increased. The micrograph shows a non-homogeneous particle distribution while the particle size and shape are uneven at low magnification. The SEM image of Laevicardium attenuatum shows an agglomeration of uneven particles. At high magnification, the particles were observed to have a brick-like structure.
 | Figure 5. SEM Micrograph of (A) Laevicardium attenuatum x 1000; (B) Laevicardium attenuatum x 2500, (C) Mytilus edulis x 700, (D) Mytilus edulis 2500 |
3.4. XRD Diffraction Patterns of Mytilus edulis and Laevicardium attenuatum
- The X-ay diffraction patterns of chitosan from Mytilus edulis and Laevicardium attenuatum are shown in Fig. 6 and 7 respectively. Different mineral phases of CaCO3 were identified in the samples. For Mytilus edulis, it was calcite and for Laevicardium attenuatum, it was aragonite. Aragonite is one of the two common, naturally occurring crystal forms of calcium carbonate, the other form being the mineral calcite. Aragonite forms naturally in almost all mollusks. Traces of Coesite (SiO2) were also found in both samples.
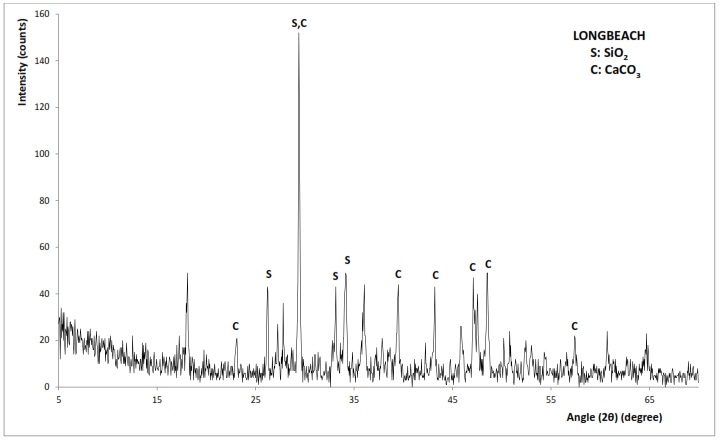 | Figure 6. XRD pattern of Mytilis edulis |
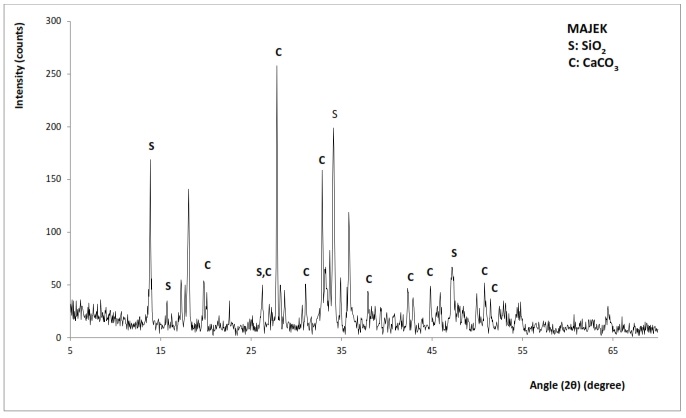 | Figure 7. XRD pattern of Laevicardium attenuatum |
3.5. X-Ray Flourescence (XRF) Patterns of Mytilus edulis and Laevicardium attenuatum
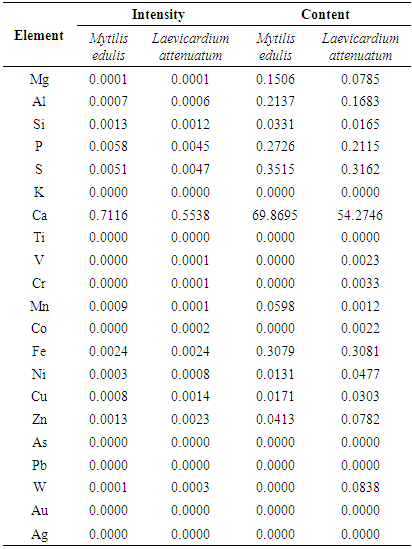
- The intensity of peaks and elemental composition of the two chitosan as revealed by the XRF are shown in Table 2. Calcium is the major element in the two chitosan. The Mytilus edulis chitosan contained 69% calcium while Laevicardium attenuatum chitosan contained 54.3%. Lead is absent in the two chitosan indicating the non-toxicity of the polymer.With an XRF spectrometer both very small concentrations of very few ppm and very high concentrations of up to 100% can be analyzed directly without any dilution process. With an XRF spectrometer, both very small concentrations of very few ppm and very high concentrations of up to 100% can be analyzed directly without any dilution process.
|
4. Conclusions
- A higher yield of chitosan with higher degree of deacetylation can be obtained from shells of Mytilus edulis. The higher degree of deacetylation could enhance the solubility of the chitosan and increase the suitability of the polymer as a permeation enhancer. The Laevicardium attenuatum chitosan with higher swelling ratio could be more suitable for sustained release formulation. Calcium is the predominant metal ion in the two chitosan. While the Mytilus edulis chitosan has a rough surface and non-homogeneous particle distribution, Laevicardium attenuatum chitosan is an agglomeration of particles which exists as a brick-like structure.
ACKNOWLEDGEMENTS
- The authors wish to acknowledge the friendly atmosphere made available to us by the technical staff in the Pharmaceutics Laboratory of the Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmacy, University of Uyo, Uyo, Akwa-Ibom State, Nigeria.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML