-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Polymer Science
p-ISSN: 2163-1344 e-ISSN: 2163-1352
2017; 7(1): 8-14
doi:10.5923/j.ajps.20170701.02

A Review of Synthesis, Characterization and Applications of Functionalized Dendrimers
Ebelegi Newton Augustus1, Ekubo Tobin Allen2, Ayawei Nimibofa1, Wankasi Donbebe1
1Department of Chemical Sciences, Niger Delta University, Wilberforce Island, Nigeria
2Department of Chemistry, Federal University Otuoke, Nigeria
Correspondence to: Ayawei Nimibofa, Department of Chemical Sciences, Niger Delta University, Wilberforce Island, Nigeria.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Dendrimers are multipurpose, nanosized particles that can be functionalized with various chemical procedures. The ability to control and engineer Critical Nanoscale Design Parameters such as architecture, shape, size, rigidity, flexibility, composition and surface chemistry offers a catalog of possibilities for utilizing dendrimers as modules for the thriving Nanotechnology industry. Contributions from researchers with various scientific backgrounds who have worked on different objectives that resulted to several applications of functionalized dendrimers are cited in this review.
Keywords: Dendrimers, Functionalization, Synthesis and applications
Cite this paper: Ebelegi Newton Augustus, Ekubo Tobin Allen, Ayawei Nimibofa, Wankasi Donbebe, A Review of Synthesis, Characterization and Applications of Functionalized Dendrimers, American Journal of Polymer Science, Vol. 7 No. 1, 2017, pp. 8-14. doi: 10.5923/j.ajps.20170701.02.
Article Outline
1. Introduction
- As part of a grand scheme to understand the evolution of natural building blocks, students of chemistry all over the world are trying to incorporate natural architectural techniques into the synthesis of compounds. This ushered in an era of macromolecules and polymers. In chemistry the term “macromolecule” means aggregates of two or more molecules held together by intermolecular forces. [1]The term ‘dendrimers’ comes from two Greek words “dendron” which means tree and “meros” which means parts, therefore referring to the typical tree-like appearance of these compounds [2]. Dendritic polymers are recognized as the fourth major architectural class of polymers after the three well known types (linear, cross-linked and branched polymers). They have grown dramatically over their about 27 years history. Presently, over 50,000 patent and literature citations related to this important class of polymers have appeared [3]. Dendritic structures are wide spread motifs in nature that are often utilized where a particular function needs to be enhanced or exposed. Dendrimers are extremely branched, globular, multivalent, mono-dispersed molecules with synthetic elasticity and many possible applications [4]. The structure of a typical dendrimer is characterized by three distinct features namely; a central multifunctional ‘core’ generations or tiers of multifunctional repeating units which are attached to the core and the terminal or end groups. Manipulating these structural features of dendrimers allows controlled synthesis of a whole series of highly branched end-functionalized macromolecules that are drawing increased attention for many potential applications [5]. The analysis of interactions between dendrimers and solid surfaces promises to provide very vital information that can expand the possibilities of using dendrimers adsorbed on solids as new adsorbents. Dendrimers can be used to modify properties of solid surfaces in order to produce composite materials that could be compactable with different kinds of environments therefore, enhancing the range of applications of dendrimer – surface supramolecule systems [6]. Because of the solvent accessibility of dendrimer voids, direct molecular design of dendrimers include; chemical modifications of their interior and periphery [7]. Rigid dendrimers seem to be suitable models for adsorption studies because they remain as solids in a wide range of temperature. Dendrimers are used in drug delivery carriers, fuel cells, light emitting diodes liquid crystals chemical modification and membrane separation [8]. In recent times the attention of most researchers and scientific investigators has been focused on the free dendrimer while the properties and applications of functionalized dendrimers (supported dendrimers) are barely reported.
2. History of Dendrimers
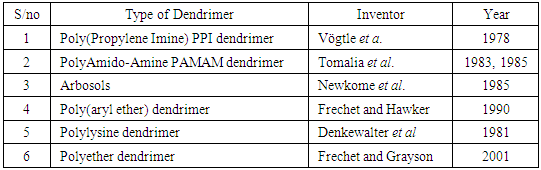
- In the design of plants and animals nature often ends up creating dendritic solutions to enhance particular properties as evidenced in the respiratory system of animals. Similarly, above the soil plants make use of their dendritic features to enhance the exposure of their leaves to sunlight also beneath the ground they have the maximum need to expose a large functional surface when collecting water from the soil. Therefore a dendrimer is both a covalently assembled molecule and a distinct nanoparticle [3]. The very first successful attempt to create and design dendritic structures by divergent synthesis was carried out by Fritz Vögtle and coworkers in 1978, followed by R.G. Denkewalter at Allied corporation in 1981, Donald Tomalia at Dow chemicals in1983 and George Newkome in 1985. In 1990 Jean Frechet introduced the covagent synthetic approach. Although a lot of researchers have concluded work in studying the different properties and applications of dendrimers but another school of thought believes the research on the properties and applications of dendrimers is still in its infancy.
|
3. Structure of Dendrimers
- Each component of a dendrimer performs a specific function and at the same time defines some other properties as it grows from generation to generation [9]. The central core acts as the molecular information hob from which shape, size, multiplicity and directionality are articulated through a covalent connectivity to the functional groups at the periphery. The Generations are the branched cell amplification region which defines the type and volume of internal void space created as the dendrimer grows. The internal voids determine the extent and nature of guest – host properties that that can be achieved in any particular dendrimer family. The surface functional groups are reactive or passive terminal groups that can perform numerous functions.
4. Synthesitic Routes
- Dendrimers are generated by changing functionality in each of their constituent parts (core, inner shell and periphery) to index properties such as solubility, thermal stability and addition of compounds for particular applications. Dendrimers are assembled from multifunctional core which is extended outward by a series of reactions called Michael addition reactions [8]. Each step of the reaction must be driven to conclusion in order to prevent trailing generations (some branches becoming shorter than others). The presence of trailing generations (impurities) can create negative impacts on the symmetry and functionality of the dendrimer, besides dendrimers are very difficult to purify because the relative size difference between perfect and imperfect dendrimers is diminutive. It is also a known fact that synthetic procedure can be applied to manage the size and number of branches on a dendrimer. There are two established methods for dendrimer synthesis, divergent and convergent synthesis, the choice of method for synthesis depends greatly on the target end application.Divergent Dendrimer SynthesisIn the very early years of dendrimers, the synthetic approach to produce the two major dendrimers (PPI and PAMAM) relied on a step by step divergent strategy. Here the creation of the dendrimer takes place in a stepwise manner which begins from the core, followed by the build- up of the molecule towards the periphery using two basic operations namely; coupling of the monomer and secondly transformation of the monomer end-group to create a new reactive surface for the coupling of a new monomer. These two steps can be repeated several times to form different generations (tiers) of dendrimers with each generation having arms from previous generations [10].
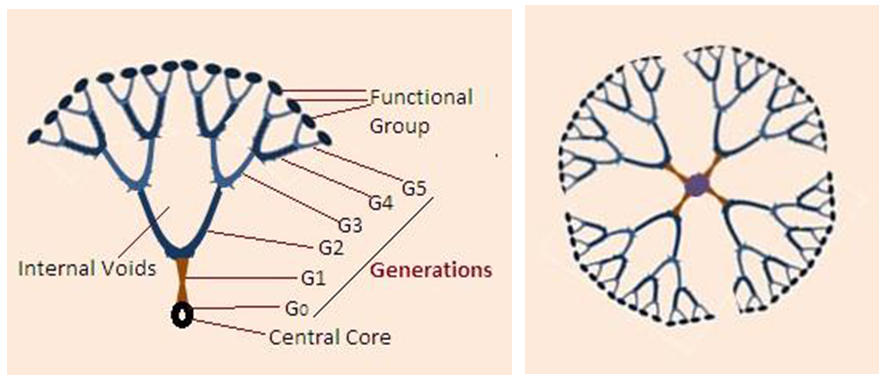 | Figure 1. Schematic representation of the Dendrimer Structure. Adopted from reference [42] |
5. Functionalization of Dendrimers
- Functionalization of dendrimer is the process of incorporating multiple active sites in dendrimers in order to create macromolecules with multifunctional architecture. Functionalized dendrimers also known as structurally controlled dendrimers have at least six well defined nanoscale features known as Critical Nanoscale Design Parameters (CNDPs) such as size, shape, surface chemistry, flexibility, rigidity, architecture and elemental composition [13].These Critical Nanoscale Design Parameters may be thoroughly manipulated to produce a wide range of new emerging properties that may be desirable and also critical for many industrial and commercial applications.Owing to systematic considerations, functionalization is usually introduced into the dendrimer framework at either the core or periphery or even both [14]. X, Shi et al., reported the crucial role played by dendrimers in the synthesis of dendrimer-stabilized Nano-Particles, the metal ion is usually complexed with dendrimer ligands such as interior tertiary amines and surface functional groups through coordination or electrostatic interactions [15]. Similarly, Harmilton et al., investigated the hydrophobic modification of G5 hydroxyl terminated PAMAM dendrimer by conjugation with dodecyl chloroformate and cholesteryl formate [16]. Welton and Stoddart also gave an account of the hydrolysis of ester-terminated PAMAM dendrimers with hydroxides of group I elements resulted in the formation of white hygroscopic powders (salts) [17].
6. Characterization of Dendrimers
- Dendrimers concern both molecular chemistry because of the step by step synthesis approach used during their synthesis and Polymer chemistry due to their repetitive structures that are made up of monomers. Therefore they are usually characterized using analytical techniques from both fields. Analytical techniques are used to investigate the chemical composition, morphology, shape, polydispersity, homogeneity, synthesis, conjugation, reaction rates, molecular weight, structural defects and purity of dendrimers [18]. They include spectroscopic methods, scattering techniques, microscopic methods, chromatographic techniques, electrical techniques and rheological / physical properties analysis.Spectroscopic TechniquesSpectroscopic analytical methods are based on measuring the amount of radiation produced or absorbed by molecular or atomic species of interest. Spectroscopic methods have provided perhaps the most widely used tool for elucidation of molecular structure as well as quantitative and qualitative determination of both inorganic and organic compounds [19].Ultar Violet – Visible Spectroscopy: This technique provides the proof of synthesis as well as surface modification on dendrimers due to characteristic absorption maximum or shift in value of lambda max (λmax). UV- Visible spectroscopy is also used to detect the functional moieties attached to dendrimer molecules. Characteristic curves in UV-Vis spectroscopy shows the specific maximum absorption peaks at specific wavelengths which is ascribed to the contribution of a conjugated moiety. Tulja et, al. reported the use of UV- Vis spectroscopy for the characterization of dendrimer – Gold Nanocomposite material [20].Infra – Red Spectroscopy: Infra-Red (IR) spectroscopy provides information for routine analysis of the chemical transformations at the surface of dendrimers [3].Thus it is an analytical technique applied for the determination of synthesis, functional groups, conjugation and drug- dendrimer interaction. Kolev et, al investigated the occurrence of hydrogen bonding in Polypropylene Imine(PPI) glycine functionalized dendrimers [21]. Similarly, Kolhe et, al. showed that the disappearance of aldehydes during the synthesis of PMMH dendrimers reflects synthesis and surface modifications [22].Nuclear Magnetic Spectroscopy: Nuclear Magnetic Resonance spectroscopy allows the determination of structure and dynamics of molecules in solution [19]. Victoria et, al. used one-dimensional and two-dimensional NMR studies to probe the conformation of melamine dendrimer which bears unique NMR signals from the core to the periphery [23].Raman Spectroscopy: Raman spectroscopy is a technique used to study vibration, rotational and other low-frequency forms in a system. This technique was applied by Furer et, al,. in the study of cyclodehydration of polyphenylene dendrimers and the characterization of polypropylene Imine(PPI) and phosphorus dendrimers [24].Fluorescence spectroscopy:Fluorescence spectroscopy is a type of electromagnetic spectroscopy which analyzes fluorescence from a sample. Fluorescence spectroscopy provides valuable information regarding the interaction between chemical additives and dendrimers. Size and shape of molecules can be determined with the help of fluorescence spectroscopy [19]. Wang and Imae reported a strong fluorescence emission from fourth generation(G4)NH2 – terminated PAMAM dendrimer upon adjusting the pH value [25].Atomic force microscopy (AFM): AFM provides a three-dimensional surface profile and better resolution. Atomic force microscopy (AFM) is a very useful technique to characterize the structure and behavior of the dendrimer agents. Pan et, al,. fabricated and characterized Polyamidoamine dendrimer modified multiwalled carbon nanotubes by AFM [26].X-ray photoelectron spectroscopy (XPS): X-ray photoelectron spectroscopy is also known as Electron Spectroscopy for Chemical analysis (ESCA). It is a quantitative spectroscopic technique utilized to measure elemental composition, empirical formula, chemical state, thickness of one or more thin layered dendrimers (1–8 nm) and electronic state of the elements that exist within dendritic framework [19]. Gates et, al,. studied the synthesis and characterization of melamine-based dendrimers [27].Microscopic TechniquesScanning Electron Microscopy (SEM): Scanning Electron Microscopy is usually applied in the study of the surface topography of dendrimers. Dadapeer et, al,. applied Scanning Electron Microscopy (SEM) in studying a phenyl-OH terminated dendrimer in order to get a deeper understanding of its surface properties [28].Transmission Electron Microscopy (TEM): Transmission Electron Microscopy (TEM) is a microscopic technique in which a beam of electrons is transmitted through an ultra-thin specimen, interacting with the specimen as it passes through it. An image is formed from this interaction and it is magnified onto an imaging device. Jackson et, al,. applied TEM in examining Poly(Amidoamine) PAMAM dendrimer molecules in order to ascertain average size, shape and size distributions for G10 to G5 [29].Electro analytical TechniquesElectro analytical techniques are capable of producing low detection limits and a lot of characterization information describing electrochemically accessible systems such as Stoichiometry and rate of interfacial charge transfer, rate of mass transfer, the extent of adsorption and the rates and equilibrium constants for chemical reactions [30]Electron Paramagnetic Resonance (EPR): This technique is applied in studying chemical species with one or more unpaired electrons such as organic and inorganic free radicals or inorganic complexes possessing transition metal ions. Ottaviani et, al. reported the use of Electron Paramagnetic Resonance in the investigation of the adsorption of dendrimers on homoporous silica, activated alumina and zeolite [7].Electrophoresis: This technique provides useful information about the purity and homogeneity of several types of water-soluble dendrimers. Ottaviani et, al. investigated the purity of Poly(Amidiamine) dendrimers with the use of gel electrophoresis, mass spectrometry and 13C NMR spectroscopy [7].Electrochemistry: In an inquiry about Copper adsorption on ChloropropylSilaca gel surface modified with Nano structured DAB – AM-16 dendrimer Carmo et. al. employed cyclic-voltametry to identify the presence of a Reduction - Oxidation process which was attributed to a Copper complex [8].TitrimetryTitrimetry is usually applied in the determination of terminal groups in dendrimers. X. Shi et. al. used potentiometric titration to determine the number of primary amine and tertiary amine groups on the surface of functionalized Poly(Amidoamine) dendrimers [31].Thermo Gravimetric Analysis (TGA)Thermo gravimetric analysis measures weight changes in a sample with respect to changes in temperature. In the same way, Differential Thermal Analysis (DTA) reveals if the changes were exothermic or endothermic. Therefore TGA-DTA measures heat flow and weight changes in a material as a function of temperature in a controlled atmosphere. Dadapeer et, al. considered the thermogravimetric analysis of a phosphorus containing dendrimer with diphenylsilanediol as core unit. The thermal stability and changes in weight with respect to change in temperature of dendrimer molecules were studied by TGA-DTA [28].
7. Applications of Dendrimer
- In view of the fact that the core, interior and surface of dendrimers can be functionalized to suit various applications, more than fifty families of dendrimers with unique properties have been developed [32]. Their unmatched molecular consistency, multifunctional surface and unique internal cavities makes dendrimers suitable for a number of wide range high technology uses as shown in Fig.2 below.
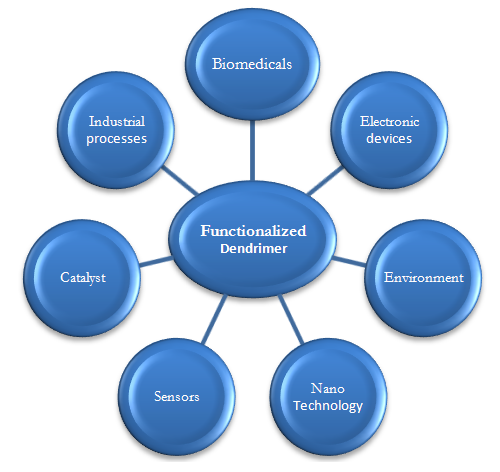 | Figure 2. Schematic representation of applications of functionalized dendrimers |
8. Conclusions
- An elevated level of control over the structural design of dendrimers has been demonstrated by the evolving tapestry of emerging techniques for the synthesis of dendrimers. Structural and functional groups can be incorporated into dendrimers in specific positions potentially giving the chemist so much control over dendritic architecture and functionality. However, more work should be done in order to make the process of functionalizing dendrimers more cost effective.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML