-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Polymer Science
p-ISSN: 2163-1344 e-ISSN: 2163-1352
2017; 7(1): 1-7
doi:10.5923/j.ajps.20170701.01

Synthesis, Characterizations and Polymerization of a Novel Fluoro-Acrylamide Monomer
S. M. Mokhtar1, F. A. Gomaa1, S. M. Abd-Elaziz1, M. Z. Elsabee2
1Chemistry Department, Faculty of Women Ain- Shams University, Cairo, Egypt
2Chemistry Department, Faculty of Science, Cairo University, Cairo, Egypt
Correspondence to: M. Z. Elsabee, Chemistry Department, Faculty of Science, Cairo University, Cairo, Egypt.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Novel Poly 4-(4'-trifluoromethyl) phenoxy acrylamide (FPAM) was synthesized. The free radical- initiated polymerization of FPAM was carried out in 1, 4 dioxane solution using azobisisobutyronitrile (AIBN) as initiator. The structure of the monomer and the polymer was confirmed by FTIR, 1H NMR, 13C NMR and elemental analysis. The effect of the monomer concentration, initiator concentration and temperature on the rate of polymerization has been investigated. The activation energy of the polymerization was calculated (ΔE= 29.5 kJ/mol). The molecular weights of Poly- FPAM 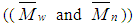 and polydispersity index of the polymer were determined by gel permeation chromatography and were found to be equal to 4.1x105, 1.3x105 and 3.2 respectively. The characterization of Poly FPAM, including thermal analysis, photo- stability, solubility and solution viscosity, were studied.
and polydispersity index of the polymer were determined by gel permeation chromatography and were found to be equal to 4.1x105, 1.3x105 and 3.2 respectively. The characterization of Poly FPAM, including thermal analysis, photo- stability, solubility and solution viscosity, were studied.
Keywords: Fluoro acrylamide monomer, Free-radical polymerization, Kinetics, Thermal analysis
Cite this paper: S. M. Mokhtar, F. A. Gomaa, S. M. Abd-Elaziz, M. Z. Elsabee, Synthesis, Characterizations and Polymerization of a Novel Fluoro-Acrylamide Monomer, American Journal of Polymer Science, Vol. 7 No. 1, 2017, pp. 1-7. doi: 10.5923/j.ajps.20170701.01.
Article Outline
1. Introduction
- Fluorinated polymers have become one of the intensive research areas due to their high thermal, chemical and weather stability, unique surface and optical properties that allowed them to be used in numerous applications. Much effort has been made to develop various fluorinated copolymers, which are applied to reduce surface tension and to decrease wetting on many types of surfaces [1-10]. Fluorine being highly electronegative has been intensively studied as a substituent affecting the structure and reactivity of carbon radicals carrying fluorine atoms [12-17]. Radical polymerization and copolymerization of some fluorinated acrylate derivatives have also been examined in terms of substituent effects due to the presence of fluorine on the monomer reactivates [18-22]. The introduction of fluorine substituent and bulky side groups into the polymer is regarded as effective ways to obtain a low dielectric constant polymers [23-25]. The incorporation of fluorine atoms (or groups containing fluorine atoms) into a polymer chains leads to increasing solubility, better thermal stability and higher glass transition temperature (Tg) of the polymers, it also leads to a decrease in moisture absorption and dielectric constant. The aromatic fluorinated polymers have currently been used as films and coatings for microelectronics devices [26-29].Acrylamide is an important chemical commodity used as coagulators, soil conditioners, and stock additives for paper treatment and in leather and textile industry [30]. Polymer of acrylamide is used as flocculants for fine solids suspended in water. Its crosslinked polymer with bisacrylamide is used as pigment retention aid in papermaking [31].Moreover, the N-substituted acrylamides are used to prepare thermo sensitive materials. In addition, these thermoplastic polymers have great potential in application as drug delivery system [31], as glycogen phosphorylase inhibitors [32], human gene vectors [33] and biocatalysts [34]. It is possible to obtain N-acryloyl and N-methacryloyl derivatives of human serum albumin (HSA), in which acryloyl fragments are bound to asparagines and lysine fragments [35]. The development of antimicrobial macromolecules holds a good promise for novel therapeutics and new materials to prevent the spread of infectious disease [36]. The reaction of acryloyl chloride or methylacryloyl chloride with the corresponding amines to prepare new functional monomers has been reported [37-40]. In this paper, a new fluoro acrylamide monomer, 4-(4`-tri fluoromethyl) phenoxy acrylamide [FPAM] has been synthesized, and polymerized by free radical mechanism.
2. Experimental
2.1. Materials
- 4-chlorobenzotrifluoride (Fluka), 4-aminophenol (Aldrich), acryloyl chloride, (E. Merck). The initiator azobisisobutyronitrile (AIBN) was recrystallized twice from Methanol (m. p. 104°C). The other reagents were used as received and the solvents were purified according to conventional methods.
2.2. Monomer Synthesis
2.2.1. 4-(4-trifluoromethyl) phenoxy aniline [28]
- In a 500 ml round bottom flask, 24.0 g (0.22 mol) of 4-aminophenol, 14.0 g (0.25 mol) of KOH and 100 ml of toluene and 200 ml of DMSO were added. The mixture was heated to 140°C with stirring under nitrogen for 3 hours followed by the removal of the produced water by azeotropic distillation with toluene. The residual toluene was distilled off and the solution was cooled to 100°C. 28 g (0.15 moles) of 4- chlorobenzotrifluoride in 40 ml of DMSO were added drop wise. The reaction was carried out with stirring at 120°C for 8 h. The mixture was then poured into 1000 ml of cold water to give pale brown crystals. The product was collected, washed thoroughly with water and then purified by recrystallization in ethanol/water. Yield 80%, m. p. 78°C. Anal. Calcd: for C13 H10 F3 N O: C, 61.5; H, 3.98; N, 5.53. Found: C, 62.16; H, 4.31; N, 5.52.
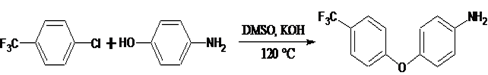 | Scheme 1. Synthesis of 4-(4-trifluoromethyl) phenoxy aniline |
2.2.2. Preparation of 4 - (4`-trifluoromethyl) phenoxy acrylamide
- 4, 4’-(trifluoromethyl) phenoxy aniline 5 g (0.0197 mole) was dissolved in diethyl ether. Acryloyl chloride 1.78 g (0.0197 mole) in the same solvent, was added drop wise. The mixture was kept in an ice bath and stirred at low temperature (-10°C). Triethylamine was added to neutralize the hydrochloric acid that is formed as secondary product. The acrylamide derivative was immediately separated as a pale brown precipitate (Scheme II). The crude product was then recrystallized twice from ethanol/water and dried in an air oven at 60°C. The features of the prepared acrylamide are as follows: (yield = 90%, m.p = 146°C). Analysis: Theoretical: C, 62.54; H, 3.77; N, 4.61. Found: C, 62.72; H 3.91; N 4.56. IR (KBr, cm-1): ν C = O; 1667, ν (Ph -O- Ph); 1242, ν N-H; 3262, ν C-H; 2930, 2859, ν C=C; 1555, (C-F) 1332. 1H NMR (300 MHz, DMSO, ppm): δ =10.24 (NH), δ 7.7 (d,2H), δ 7.14 -7.09 (d, 2H), δ 6.4 (d, CH), δ 5.74 - 6.296 (d, CH2), 13C NMR (125 MHz DMSO): δ 163.59, 161.4 (CO), 150.78 (COC), 136.5, 132.28 (ethylenic, CH), 127.89, 127.45 (CF3), 121.6 (2CH, Ar), 121.1 (2CH, Ar), 117.88 (2H, Ar).
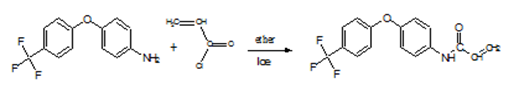 | Scheme 2. Synthesis of N-[4- (4`-fluoromethyl phenoxy)] acrylamide (FPAM) |
2.3. Analytical and Spectroscopic Methods
- The FTIR spectra of the prepared monomer and its polymer were recorded by I.R spectroscopy (4100 JASCO FT/IR). 1HNMR Spectra were measured in DMSO for monomer and polymer with tetramethyl silane as internal standard on Varian Mercury VX-300 NMR spectrometer. 1H NMR spectra were run at 300 MHz whereas, 13C NMR spectra were recorded in DMSO for FPAM on JEOL- ECA-500. 13C NMR spectra were run at 125 MHz. The X-ray analysis was carried out using X-ray diffraction instrument Philips Pw 1390 channel control. Cu Target Kα = 1.542, Ni filter is 40 Kv, 25 mA. The viscosity measurements were carried out using an Ubbelohde suspended level dilution viscometer. Dioxane was used as solvent with a flow time of 121 seconds at 25°C. The gel permeation chromatography (GPC) of the samples was performed using 1100 Aligant instrument equipped with organic GPC- SEC start up kits with a flow rate of 1 ml /min, THF as mobile phase, maximum pressure 150 bar, minimum pressure 5 bar, injection volume 20µL and column temperature thermostat 25°C. The eluent was monitored with a refractive index detector of optical unit temperature 25°C and peak width 0.1 min. Polymer concentration was 0.1 (Wt/%). The thermogravimetric analysis of the PFPMI powder was carried out in nitrogen atmosphere with a heating rate of 10°C min-1 from room temperature and up to 700°C by Shimadzu TGA – 50 H. The glass transition (Tg) temperature was measured by differential scanning calorimeter analysis, (DSC) using Shimadzu DSC-60 with a heating rate of 10°C /min under nitrogen atmosphere.
3. Results and Discussion
3.1. Monomer Synthesis
- A novel acrylamide monomer FPAM has been synthesized in a manner presented in the experimental part. The monomer structure was confirmed by elemental analysis, FTIR, 1H-NMR and 13CNMR “Figure 1(a-c).”
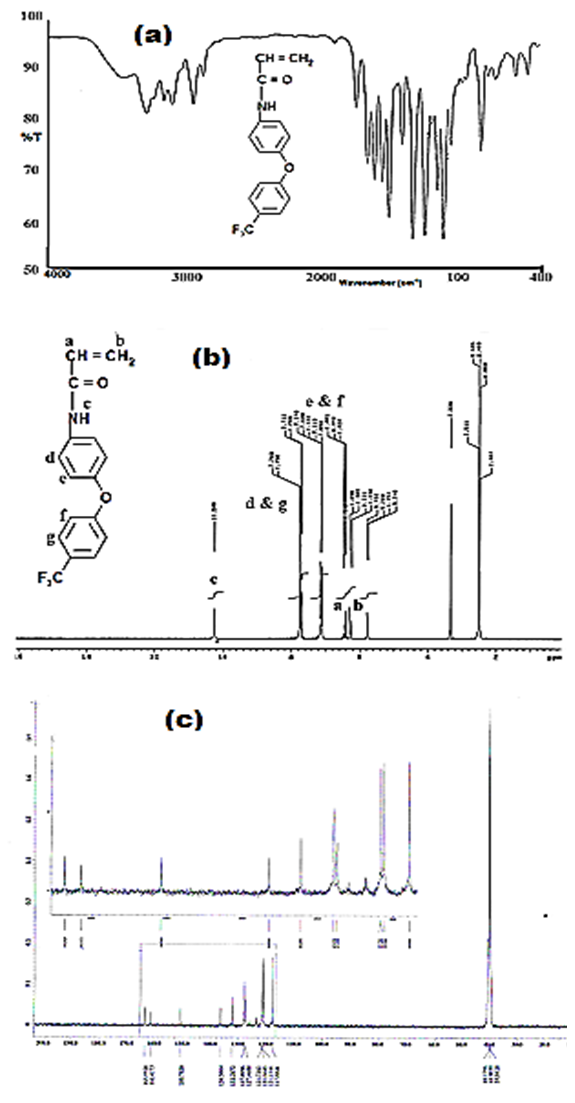 | Figure 1. FTIR (a), 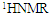 (b), and (b), and 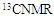 (c) spectra of FPAM (c) spectra of FPAM |
3.2. Polymerization
- FPAM was polymerized in 1, 4-dioxane using AIBN as initiator at 75°C ± 0.1. Polymerization was carried out in calibrated dilatometers (3-5 ml in capacity) with ground joint stoppers. The polymerization reaction proceeded homogenously. The polymers were purified by re-precipitation from dioxane solution into ethanol. The FTIR spectrum of the prepared polymer showed the disappearance of the band at 1555 cm-1, which is due to the stretching vibration of the C=C bond. This confirms the polymerization of FPAM which has occurred by the opening of the C=C of the acrylamide group “Figure. 2”. The FTIR spectrum of the prepared polymer shows the sharp peaks at 1237 cm-1 and 1328 cm-1 due to the presence of CO (acrylamide) and ph-O-ph (phenoxy) groups, respectively. Peak at 1328 cm1 is due to the CF group and the peaks at 2929 cm-1 and 833 cm-1 are assigned to the CH group. “Figure. 2a”. The 1H-NMR spectra of the polymer further confirm the structure. The 1H NMR spectra of the polymer shows the band at δ= 9.8 ppm (s, NH), broad band characteristic of the aromatic protons of polymeric material at δ=7.5-6.86 ppm and the bands at δ= 3.5- 4.5 ppm of (s, CH- 2H). The disappearance of the band at δ= 6.4 ppm, which is due to the double bond, is another indication that polymerization has occurred “Figure 2b”.The rates of free radical polymerization for FPAM were measured dilatometrically in solution. The kinetic parameters of polymerization of the FPAM were investigated by determining the order of reaction with respect to the initiator concentration [I] and monomer concentration [M] as well as the activation energy of the polymerization process.
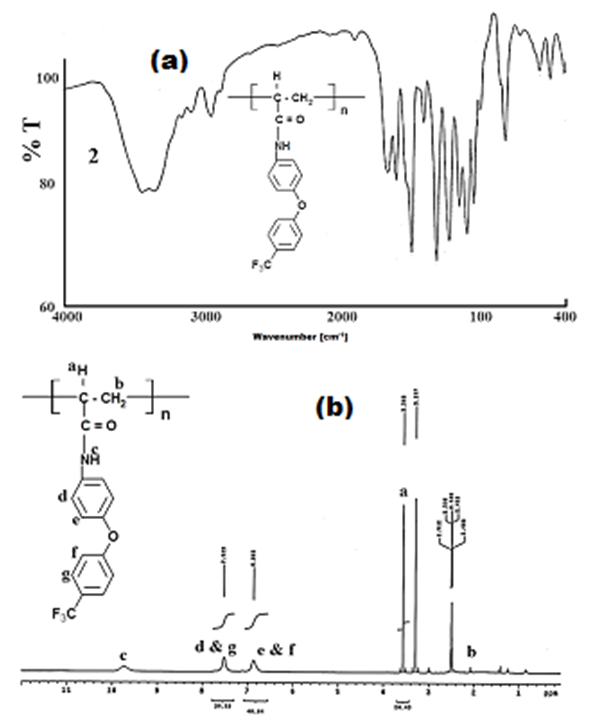 | Figure 2. FTIR (a), and 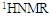 (b) spectra of Poly- FPAM (b) spectra of Poly- FPAM |
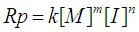 | (1) |
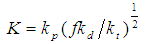 | (2) |
3.3. Effect of Monomer and Initiator Concentration on the Polymerization of FPAM
- The extent of polymerization is proportional to the decrease in the volume of polymerization medium with time. The initial rates of polymerization (Rp) has been determined from the slope of the decrease in volume vs time. By plotting the logarithm of the rate of polymerization log [Rp] versus the logarithm of the monomer concentration log [M] at constant initiator concentration [I], a straight line is obtained with a slope equals to the order of reaction regarding the monomer concentration as 1.12 “Figure. 3”. In a similar manner, the order of initiator concentration was measured and equals to 0.56 at constant monomer concentration equals to 0.6 mol.L-1 “Figure. 4”. Consequently, the rate of polymerization equation is Rp= k [M] 1.12 [I] 0.57 indicating a typical free radical mechanism.
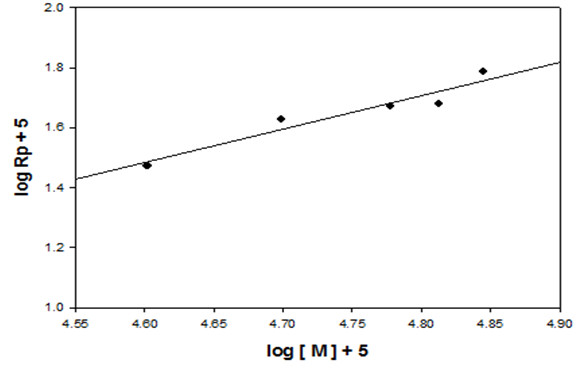 | Figure 3. Effect of monomer concentration on the rate of polymerization in THF, the initiator concentration 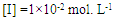 and at 75°C and at 75°C |
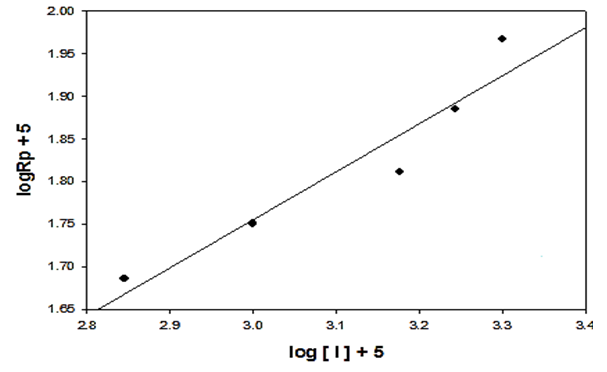 | Figure 4. Effect of initiator concentration on the rate of polymerization in THF, the monomer concentration 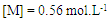 and at 75°C and at 75°C |
3.4. Effect of Temperature
- The effect of temperature on the rate and degree of polymerization is of prime importance. Increasing the reaction temperature usually increases the polymerization rate and decreases the polymer molecular weight. “Figure 5” shows the effect of temperature on the rate of polymerization at constant monomer and initiator concentration. The activation energy of the polymerization can be calculated based on Arrhenius –type equation,
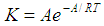 | (3) |
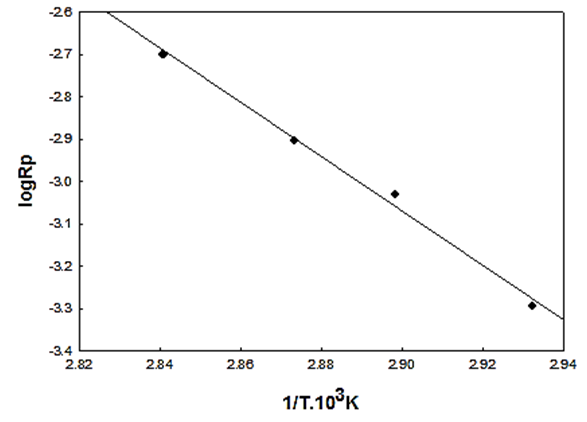 | Figure 5. Effect of temperature on the rate of polymerization in THF, 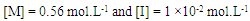 |
3.5. Molecular Weight Determination
- The intrinsic viscosity of the prepared polymer was measured in dioxane at 25°C using Ubbelohde viscometer. “Figure. 6” shows that the intrinsic viscosity [η] increases with increasing monomer concentration and decreases with increasing the initiator concentration, which is a well expected behaviour for free radical polymerization. The weight average
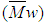 , number average
, number average 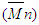 molecular weight and polydispersity index
molecular weight and polydispersity index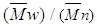 of polymer sample were obtained from gel permeation chromatography. The values of number average and weight average molecular weight of Poly-FPAM are equal to 1.3 x105 and 4.1x105 respectively. The polydispersity index of the Poly-FPAM is equal to 3.2. These data reflects the high molecular weight of the prepared polymer.
of polymer sample were obtained from gel permeation chromatography. The values of number average and weight average molecular weight of Poly-FPAM are equal to 1.3 x105 and 4.1x105 respectively. The polydispersity index of the Poly-FPAM is equal to 3.2. These data reflects the high molecular weight of the prepared polymer.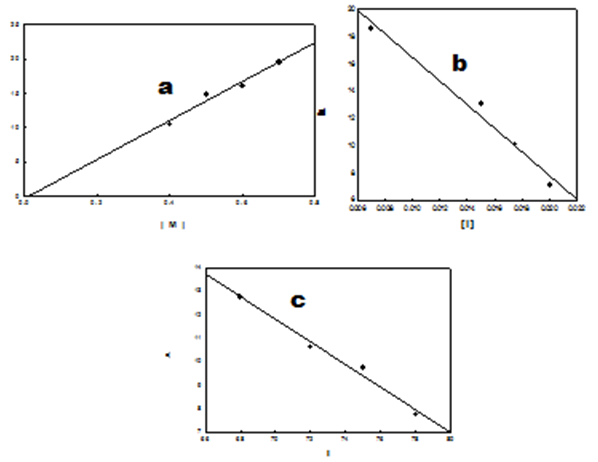 | Figure 6. Effect of: monomer concentrations (a) initiator concentrations (b) and temperature (c) on the intrinsic viscosity of Poly- FPAM |
3.6. Characterization
3.6.1. X-Ray Diffraction
- The x-ray method allows the calculation of the relative amounts of crystalline and amorphous percent in a polymer. The crystallinity of Poly-FPAM was examined by X-ray diffraction. The X-ray diffraction pattern of the prepared polymer exhibits an amorphous shape [43]. These results could be explained by the presence of bulky pendant groups, which disrupted the regularity of the molecular chains and inhibited the close packing of the polymer chains “Figure. 7” it is also expected from the free radical polymerization method which usually leads to amorphous polymers.
 | Figure 7. The x-ray diffraction of: FPAM monomer (a) and Poly- FPAM (b) |
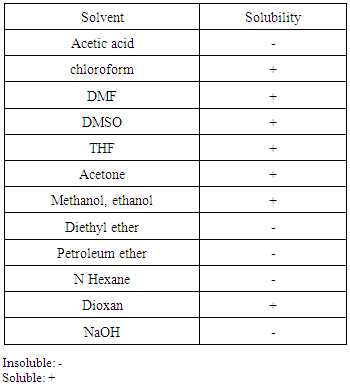
3.6.2. Solubility
- Table (1) depicts the solubility data of the new Poly-FPAM in some organic solvent and organic acid. The data indicate that Poly-FPAMs are soluble in some organic solvents including non-polar solvents. The excellent solubility of PFPAM could be attributed to the presence of bulky pendant groups and fluorine atoms into the polymer which led to disturbing the regularity of molecular chains and increasing the free volume.
|
3.6.3. Thermal and Photo Properties
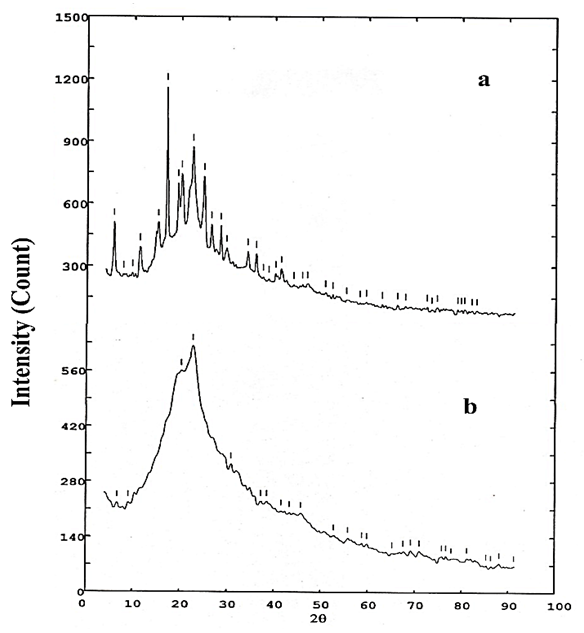
- Thermogravimetric analysis was used to evaluate the thermal stability of Poly-FPAM. The initial decomposition temperature (Tinit) and the maximum decomposition temperature (Tmax) were determined by thermogravimetric analysis. “Figure 8” shows the thermogram for PFPMI in nitrogen at a heating rate of 10°C/min. The diagram shows that there are three stages of weight loss. The first stage starts at about 100°C with a maximum decomposition temperature at 129°C. This may be due to the presence of moisture and solvent. In the second stage, the minimum decomposition temperature is at 170°C, while the maximum decomposition temperature occurs at 440°C. This stage could be due to chain scission. In the third stage, the minimum decomposition temperature was at 441°C, while the maximum decomposition temperature was at 649°C. The TGA curve shows that Poly-FPAM has excellent stability similar to the other fluoro and maleimide polymers [28].
 | Figure 8. Thermogravimetric and the differential curves of Poly-FPAM |
4. Conclusions
- Trifluoromethyl phenoxy Acrylamide monomer was synthesized and characterized. The free radical polymerization of FPAM was initiated by AIBN in 1,4dioxane. The characterizations of the polymer as well as the rates of free radical polymerization were studied. The activation energy ΔE of the polymerization was calculated and it was found to be equal to 29.5 k J/ mol. The FPAM polymer has a high molecular weight. The average molecular weight (
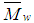 and
and 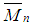 ) and polydispersity index are equal to 411,061, 129,046 and 3.185 respectively. The Poly-FPAM bosses high glass transition temperature (Tg = 285.68). Also, the polymer exhibits a good solubility, high thermal stability and high glass transition temperature as well as photo stability.
) and polydispersity index are equal to 411,061, 129,046 and 3.185 respectively. The Poly-FPAM bosses high glass transition temperature (Tg = 285.68). Also, the polymer exhibits a good solubility, high thermal stability and high glass transition temperature as well as photo stability. Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML