-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Polymer Science
p-ISSN: 2163-1344 e-ISSN: 2163-1352
2016; 6(3): 86-91
doi:10.5923/j.ajps.20160603.04

Current Development of Extraction, Characterization and Evaluation of Properties of Chitosan and Its Use in Medicine and Pharmaceutical Industry
Stephen O. Majekodunmi
Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmacy, University of Uyo, Uyo, Nigeria
Correspondence to: Stephen O. Majekodunmi, Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmacy, University of Uyo, Uyo, Nigeria.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Chitin and chitosan are waste products of sea shells, shrimps and prawns when the internal mollusks have been eaten or are dead and these sea shells are mostly scattered along many of the sea coasts constituting a nuisance. This review from various literatures explains the methods of extraction, evaluation of properties of chitosan obtained from sea shells for use in the pharmaceutical industries. Chitosan is obtained from chitin by deacetylation to a higher greater degree of 70 – 90% taking advantage of their biocompartibility and biodegradability. Advance research works of chitosan are also discussed. Biopolymer like chitosan is commanding a new research initiative partly due to its biodegradability, biocompartibility and their biological and physicochemical properties. Researchers take advantage of these properties in its potential application in medicine, pharmaceutics and biotechnology.
Keywords: Chitin, Chitosan, Deproteinization, Demineralization, Deacetylation, Pharmaceutical applications, Biological activities
Cite this paper: Stephen O. Majekodunmi, Current Development of Extraction, Characterization and Evaluation of Properties of Chitosan and Its Use in Medicine and Pharmaceutical Industry, American Journal of Polymer Science, Vol. 6 No. 3, 2016, pp. 86-91. doi: 10.5923/j.ajps.20160603.04.
Article Outline
1. Introduction
- Chitosan is a modified natural carbohydrate polymer derived from chitin which has been found in a wide range of natural sources such as sea foods, shrimps, crustaceans, fungi, insects and some algae [1]. The shell of selected crustacean consists of 30-40% protein, 30-50% calcium carbonate and calcium phosphate, and 20-30% chitin. Chitosan is a homopolymer of ß-(1→4)-linked N-acetyl-D-glucosamine while chitin is a linear chain of acetylglucosamine groups and chitosan is obtained by removing enough acetyl groups (CH3-CO) and this is soluble in most diluted acids. In essence, the actual difference between chitin and chitosan is the acetyl content of the polymer. Chitosan is very reactive because of its free amino group [2].Chitosan is a non-toxic, biodegradable polymer of high molecular weight. Unlike plant fiber, chitosan possesses positive ionic charges (the amine group), which give it the ability to chemically bind with negatively charged fats, lipids, cholesterol, metal ions, proteins, and macromolecules [3]. Chitosan has attained increasing commercial interest as suitable resource materials due to their excellent properties including biocompatibility, biodegradability, adsorption, and ability to form films, and to chelate metal ions [4]. Consequently, chitosan is useful in a wide application in various industries such as pharmaceuticals, biochemistry, biotechnology, cosmetic, biomedical industries. Nanoparticles, microspheres, hydrogels, films, and fibers are typical chitosan based forms for biomedical and pharmaceutical applications. Examples of such applications include nasal, ocular, oral, parenteral and transdermal drug delivery [5].One of the objectives of this review is to determine optimal condition of chitosan production from shrimp processing waste and to investigate the characteristics properties of chitosan and the previous works on chitosan.
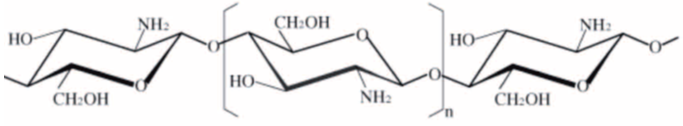 | Figure 1. Chemical structure of chitosan |
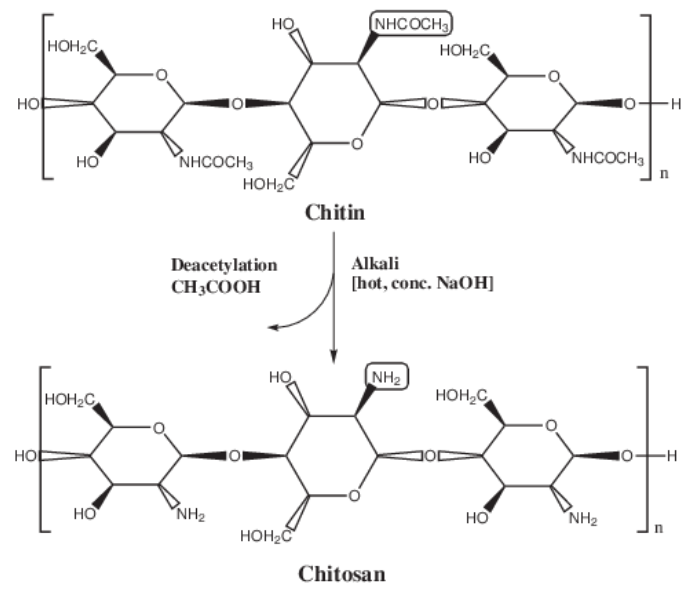 | Figure 2. Chemical structure of chitosan and its production from chitin |
2. Extraction of Chitosan from Shrimp Shell Waste
- Most of the methods for extraction of chitosan from shrimp shell waste are laborious and time consuming or low yielding. The most simple and effective method of extraction of high purity yield chitosan from shrimp shell waste is that which is prepared using a combination of three procedures [6], [7], [8]. A specified quantity of ground (250 microns) shrimp or oyster shell waste is treated with 4% NaOH at room temperature for 24hours. The alkali is drained from the shell mixture and washed with distilled water repeatedly till pH dropped to neutral. This processed is called deproteinization of shells. The deproteinized shells again are treated with 4% HCL at room temperature for 12 hours for demineralization to yield chitin. The acid is drained off from chitin, washed with distilled water and finally dried at room temperature. This is repeated with 2% NaOH and % HCL. The chitin obtained may still have a slight pink colour. Further decolorization is achieved by soaking chitin in 1% oxalic acid for 30 minutes to 2 hours. The decolorized chitin is deacetylated to form chitosan by treating with 65% NaOH for 3 days at room temperature. Alkali is drained off and washed repeatedly with distilled water till pH is lowered. Chitosan obtained is further dried at room temperature and stored in airtight container [9].
2.1. Alternatives to Traditional Chemical Extraction of Chitosan
- The chemical process described above is expensive and environmrntsl friendly. Biotechnological production of chitosan through fermentation though not commercially available offers respite for the production of highly viscous chitosan [10] which can be used in medicine and pharmacy [11], [12]. Fermentation of shells uses lactic acid producing bacteria for the production of chitin has been studied and reported [13].
3. Characterization of Chitosan Properties - Estimation of Chitosan Yield
- The weight of chitosan produced is measured and yield calculated. This method of chitosan extraction is superior to other known methods based on the higher yield expected of pure quality chitosan. Deproteinization and demineralization steps are repeated twice. This aided in higher yield of chitin from the shells. The final deacetylation of chitin at room temperature for 3 days gives a longer reaction time which may result in higher yield of chitosan [9].
3.1. Composition Analysis
- Moisture content and residue on ignition or ash content are analysed based on methods by Association of Oficial Analytical Chemists. The moisture content may vary depending on season, relative humidity and intensity of sunlight [14]. The ash content of chitosan is an indication of the effectiveness of the method employed for removing inorganic materials [14].
3.2. pH
- The pH measurement of chitosan solutions is carried out with the use of a pH meter.
3.3. Viscosity
- Viscosity is determined using a viscometer [15]. Viscosity of chitosan can be used to determine molecular weight. High molecular weight chitosan yields high viscous solution. Hence low viscosity is more preferred [16].
3.4. Degree of Deacetylation
- Chitosan solution is prepared using dilute HCl containing 0.01 mol/L and titrated against 0.1M NaOH. The end point is detected by the inflection of pH values. Two inflections are mainly noted. First one corresponds to HCl neutralization and second to neutralization of ammonium ions of chitosan. The difference between two points gives the amount of amino groups in chitosan chain which also called degree of deacetylation. The higher the degree of deacetylation the higher the amount of protein [6].
3.5. Solubility of Chitosan
- The solubility of chitosan in acetic acid is a mark of its purity. Chitosan dissolves completely in 1% acetic acid. A few grams of chitosan and 35mL 1% acetic acid is weighed. The solution is kept in a magnetic stirrer for 30 minutes. The sample is taken out and the insolubles are removed by filtration through Whatmann No. 1 filter paper and weighed. For example, if the concentration of chitosan in acetic acid is 7.7g/L, it indicates that the obtained chitosan is 77% pure. Chitosan, unlike chitin has high content of highly protonated free amino group that attracts ionic compounds. This is why chitosan is soluble in inorganic acid [17].
3.6. X ray Diffraction Spectrometer (XRD)
- X ray diffraction analysis of chitosan is used to detect its crystallinity. The XRD pattern will show characteristic peaks at 2θ = 9.28 and 20.18°. The sharper peaks will be an evidence of denser crystalline structure. However, the characteristics peaks of chitosan is reported in range of 2θ = 9.9 – 10.7 and 19.8 – 20.7 [18].
4. Pharmaceutical Use of Chitosan
4.1. Delivery Drug Carrier
- Water in oil emulsion is used in preparing chitosan microspheres as delivery drug carriers by solvent diffusion method, ethyl acetate being the oil phase. Figure 3 displays Scanning Electron Microscopy (SEM) image of the chitosan microspheres with drug entrapment [19]. Chitosan has been largely employed in many areas such as biotechnology, biomedical products (artificial skin, wound dressing, contact lens, etc.), system of controlled liberation of medicines (capsules and microcapsules) [19].
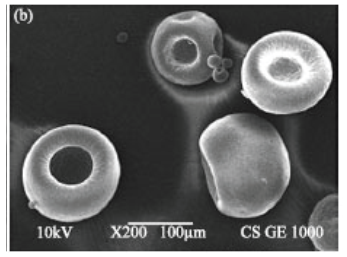 | Figure 3. Scanning Electron Microscopy (SEM) of drug-loaded chitosan microparticles (reproduced from Phromsopha & Baimark, 2010) |
5. Advance Research Works on Chitosan
5.1. Characterization of Chitosan Molecular Weight
- Characterization of chitosan molecular weight is achieved by aqueous size exclusion chromatography coupled with multi-angle laser light scattering (SEC–MALLS) to supply absolute average molecular weight, Mn, weight-average molecular weight, Mw, and polydispersity index, PDI = Mw/Mn. TSK-gel PW and PWXL columns from Tosoh Bioscience are often used for the molecular weight characterization of chitosan by SEC [20], [21], [22].
5.2. Thermal Analysis
- Thermal analysis is widely used for the characterization of polymers [23]. Among the techniques are thermogravimetry (TGA), differential scanning calorimetry (DSC), and dynamical mechanical thermal analysis (DMTA). El-Hefian et al, [24], investigated the rheological and thermal studies of chitosan in acetic acid using the influence of temperature, concentration, shearing time, and storage time on the rheological properties, i.e. the dynamic viscosity and shear stress, as a function of shear rate of chitosan solubilized in weakly acid solutions. They found out that the shear thinning behavior (pseudoplastic non-Newtonian behavior) was pronounced at temperatures from 20 to 50°C, but was more remarkable at lower temperature. Furthermore, an increase in viscosity was obtained with extending the period of storage to 3 months, after which a drop in viscosity was recorded. Thermal properties of chitosan films are also investigated by employing thermogravimetric analyses (TGA) and differential scanning calorimetry (DSC) [24]. The study concluded that the dynamic rheological measurements of chitosan solutions in acetic acid suggested pseudoplastic non-Newtonian behavior. The shear thinning behavior was remarkable at temperatures between 20 and 50°C. Chitosan solutions in acetic acid were found to obey the Arrhenius equation. In addition, chitosan in acetic acid solutions exhibited less shear thinning and an increase in viscosity with increasing concentration. This study has also shown that curves of the dynamic viscosity of chitosan solutions show similar behavior at all shearing times of 15-75 sec and that less shear thinning behavior and higher values of viscosity of chitosan solutions were observed when the storage period was extended to 3 months. A general increase in viscosity with time was also observed at a constant shear rate, suggesting rheopexy behavior. However, a drop in viscosity was recorded in the fourth month. On the other hand, DSC results of chitosan film showed agreement with those obtained by TG measurements concerning the thermostability and degradation [24].
5.3. Antimicrobial Properties of Chitosan
- Limam et al., [25] tested microorganisms on two Tunisian crustaceans species. These were Parapennaeus longirostris waste and Squilla mantis. The microorganisms used were strain of bacteria Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus, four fungi Candida glabrata, Candida albicans, Candida parapsilensis and Candida kreusei. Their result showed that Squilla chitosan had a minimum inhibitory concentration (MIC) against the different fungi exceptionally for C. kreusei. The antioxidant activity was investigated with 2, 2-diphenyl-1-picrylhdrazyl (DPPH). Parapennaeus longirostris chitosan showed the highest radical scavenging properties [25]. Chitosan oligomers are known to have various biological activities including antitumor activities [26], immune-enhancing effects, protective effects against infection with some pathogens anti-fungal activities and antimicrobial activities [27]. Chitosan can inhibit the growth of a wide range of bacteria. This is due to the fact that chitosan possesses a high antibacterial activity, a broad spectrum of activity, a higher killing rate, and lower toxicity toward mammalian cells.
5.4. Solubility of Chitosan
- The N-deacetylated derivative of chitin, chitosan is insoluble at neutral and alkaline pH, but soluble in inorganic and organic acids such as hydrochloric, glutamic acids; acetic, formic and lactic acids [28]. The free amino group is responsible for chitosan’s chemical and biochemical reactivity. The properties that make chitosan commercially relevant are its biodegradability, biocompartibility and the ability to transform into gels, beads, fibres, colloids, powders and capsules [28]. These attributes have given chitosan its much attention as a functional biopolymer for diverse applications in pharmaceuticals, foods and cosmetics.
6. Application of Chitosan
- Natural and non-toxic biopolymers such as chitosan are now widely produced commercially from crab and shrimp shell waste. During the past few decades, chitin and chitosan have attracted significant interest in view of a wide range of proposed novel applications. Their unique properties, biodegradability, biocompatibility and non-toxicity make them useful for a wide range of applications. Chitin is mainly used as the raw material to produce chitin-derived products, such as chitosans, oligosaccharides, and glucosamine [29]. The worldwide industrial production of these derivatives in year 2000 is estimated to be above 10 000 tonnes [30].
6.1. Wastewater Treatment with Chitin and Chitosan
- Chitosan is used for the adsorption or fixation of heavy metals and dyes and in immobilization of microorganisms or sludge in chitosan matrices for waste water treatment in extreme environmental conditions. Chitosan is also effective in coagulation, flocculation and dehydration of activated sludge for wastewater treatment [30], [31].
6.2. Biomedical Application of Chitosan
- Because of chitosan’s high biocompartibility, biodegradability in physiological environment [32] it shows excellent biological properties such as non-toxicity [5], [33]; biodegradation in the human body [34], [35], [36], biocompatibility [37], [38], and immunological, antibacterial, wound-healing [39] and haemostatic activity [40], in cell culture, tissue engineering [41], [42], [43], and drug delivery [44].
6.3. Chitosan Blends
- Chitosan blends with natural polymers [45], [46] or synthetic polymers [47] have been reported [48]. Miscibility, structure and properties of the constituents of the blend is a key factor influencing the properties of a polymer blend. New materials and properties normally evolve from the chitosan blend when the original polymers are compartible. By synergistic effects the blend provides better properties than the pure component [49].
7. Conclusions
- The extraction, evaluation, characteristics and properties of chitosan has been described with a view to showcase the importance of chitosan in medicine and pharmaceutical industries. The relevance of chitosan resides in tis biological activities of biodegradability, biocompatibility, non-toxicity and physicochemical properties of degree of deacetylation and molecular mass. In the chemical extraction of chitosan from chitin high temperature is better avoided because heat can destroy the physicochemical properties of the polymer. New materials with better activity are observed when the original polymer is compatible and the resulting blend provides better properties than the pure compounds. There is no doubt that chitosan remains one of the most important biopolymer.
ACKNOWLEDGEMENTS
- The author wishes to thank the various authors who have worked on chitin and chitosan and made their online publications accessible to me as a literature review. In this regards many thanks to D. Sakthivel et al., Wassila Arbia et al., Divya et al., M.S, Islem Younes and Marguerite Rinaudo, Elso Santiago de Alvarenga, Zouhour Limam et al. etc.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML