-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Polymer Science
p-ISSN: 2163-1344 e-ISSN: 2163-1352
2016; 6(3): 68-74
doi:10.5923/j.ajps.20160603.02

Radiation Crosslinking of Neoprene W with Diallyl Etherofmaleic Acid and Epoxy Resins
Sh. M. Mammadov1, A. A. Asadova1, R. F. Khankishiyeva1, O. H. Akbarov2, G. Sh. Duruskari2, M. N. Maharramov2, J. M. Mamedov1, H. N. Akhundzada1
1Institute of Radiation Problems of ANAS, Azerbaijan
2Baku State University, Azerbaijan
Correspondence to: Sh. M. Mammadov, Institute of Radiation Problems of ANAS, Azerbaijan.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Discusses the use of technological aspects and radiation crosslinking Neoprene W in the presence of a diallyl ester of maleic acid (DAEMA) and epoxy resin (ED-5) in the presence of metal oxides. With the help of physico-chemical and spectral methods shows the variation in the molecular structure Neoprene W in the presence of DAEMA and ED-5 after irradiation with γ-rays of 500 kGr. With analysis method of Sol-gel for each test system determined the radiation-chemical yield (RCY) and the emergence of cross-linking of cross-links in the elastomer. The dependence of the crystallinity index of the degree of stretching for Neoprene W, irradiated at 500 kGy was defined. It was found that at the radiation vulcanization Neoprene W in the presence of a bifunctional bonds epoxy resin weakly has a decisive influence on the kinetics of the process and yield crosslinking. It is shown that above 1500 kGy irradiation in the filled Neoprene observed destruction in the elastomer chain, resulting in deterioration of physical and mechanical properties of the vulcanizates.
Keywords: Neoprene, Elastomer, Vulcanization, Radiation, Allyl, Sensitizer, Gel, Crosslinking, Dose, Crosslinks
Cite this paper: Sh. M. Mammadov, A. A. Asadova, R. F. Khankishiyeva, O. H. Akbarov, G. Sh. Duruskari, M. N. Maharramov, J. M. Mamedov, H. N. Akhundzada, Radiation Crosslinking of Neoprene W with Diallyl Etherofmaleic Acid and Epoxy Resins, American Journal of Polymer Science, Vol. 6 No. 3, 2016, pp. 68-74. doi: 10.5923/j.ajps.20160603.02.
Article Outline
1. Introduction
- Neoprene W (DuPont, France) is one of the most promising elastomers for special purposes. This is due to its valuable properties high physical and mechanical properties. [1-5].Neoprene W differs considerably greater tendency to radiation crosslinking than other diene elastomers. It vulcanized under irradiation even without additives and auxiliaries, and in the presence of low molecular weight additives can be prepared valuable technical vulcanizates [6-10].Radiation-chemical synthesis of Neoprene W in a process homogenous vulcanization, as a rule, complicated kinetic activity in the reaction of the polymer chains [11-14].At the crosslinking a Neoprene W exposure to ionizing radiation [15-17] as well as in their mixtures with small amounts of poly functional monomers as the allyl-methacrylate leads to the formation of high molecular weight polymer.Due to the fact that the Neoprene W is an unsaturated elastomers, proceeding from the structure of Neoprene W and reactivity abilities sheet polymer molecules in principle carried thermal and radiation vulcanization with help a fairly wide range of substances. The high molecular Neoprene W is also irradiated with the participation of low molecular weight organic sensitizers, triazine bondss. The activity of these sensitizers in radiation-chemical processes is low, probably due to the weak influence of the properties [14, 18, 19].It is known that for achievement optimum properties vulcanizates requires a fairly large dose, which increases the cost, prime cost of materials and serves possibility of using radiation instead of thermal. In this regard, very relevant is the question of how reduce the radiation dose methods, necessary for obtaining optimum properties and hence improve the performance of the process [14, 17, 19, 20].Neoprene W is a convenient object for studying the effect of the sensitizing agent which has active functional allyl group also cyclic modifier has cycle epoxide groups, which allows us to estimate the effect of these functional groups on the parameter space grid radiation vulcanizates.Effect of contents allyl and epoxy groups to study the structure and properties of Neoprene W depends not only on the magnitude of the absorbed dose, and the activity of the solid oxide additives [21, 22].The mechanism of radiation crosslinking Neoprene W in these crosslinking systems is still insufficiently understood.The results of investigation of the impact sensitizer diallyl ether of maleic acid (DAEMA) and epoxy resin modifier on the kinetic and structural parameters of the grid vulcanizates exposure to ionizing radiation are set out in the article.
2. Methods and Object of Research
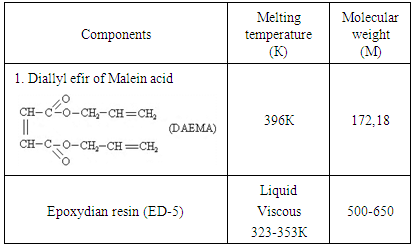
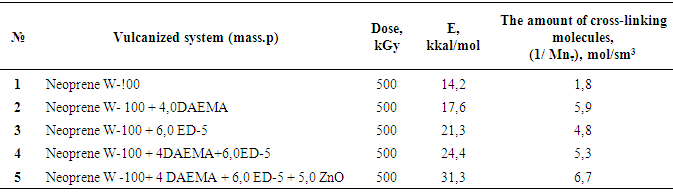
- For radiation curing used chloroprene rubber stamp Neoprene W (French product) obtained by emulsion polymerization. By results of Fourier research spectroscopy isomeric composition of the double bonds in the investigated polymer 1,4-trans units consists 86.5%, cis 1,4-10%; 1.2-1.6% and 3,4-1%.Crystallizability plays a big important role in the recycling of Neoprene W. Maximum speed of crystal-lization amounts 263K.Neoprene W macromolecules consist of static distribution links of butadiene and vinyl chloride. Especially in radiation chemical cross-linking Neoprene W, affect their microstructures, molecular weight, MWD (molecular weight distribution) and the gel content. That is why, prior the radiation-chemical processes in the preliminary elastomer viscosimetric method and the gel permeation chromatography (GPC) determined average and weight average molecular weight and the polydispersity (Mn = 72 231 = th. Mw=231 th.Mw:Mn = 3.2). For initiate the chemical processes used radiation sensitizer diallyl ester of maleic acid (DAEMA). DAEMA is prepared by reacting maleic anhydride with allyl alcohol in the presence of sulfuric acid (M = 182, melting point= 385K, white liquid, soluble in aromatic hydrocarbons) One of the most important conditions for the normal flowing of radiation crosslinking Neoprene W is to provide the required molecular weight and yield of crosslinks.Proceeding from the structure of the isoprene rubber and the reactive sites of the polymer molecules, in principle, crosslinking can carry out by using a synthetic epoxy resin. Naturally that efficiency of crosslinking process will determine overlooking Neoprene W rubber and concentration necessary for given type of vulcanizing system reactive places and type of crosslinking system.Epoxy resins are linear polyethers, at the ends of molecular chains which are capable of highly reactive groups, and chains secondary hydroxyl groups.The mainly part of epoxy resin received in the laboratory or industrial scale constitute resin formed when interacting of polyphenols with epichlorohydrin.For receipt with the purpose of the activation process and the yield of crosslinking introduced into heterogeneous systems, active zinc oxide was evaluated by band gap (∆E=3,3eV) thereby found practical application and dosage which are selected strictly in accordance with the individual characteristics at radiolysis. For holding of crosslinking have been used for 100 mas.p. of elastomer (Table 1).
|
|
3. Results and Discussion
- Despite the large number of studies on the effects of ionizing radiation on the elastomers, it is still not yet sufficiently clear mechanism of crosslinking Neoprene W quasi-binary systems. Furthermore, in the literature there are conflicting data on the assessment of output crosslinking loss of unsaturated and other structural changes in Neoprene W by irradiation.Reducing the intensity of the absorption bands specific to the studied sensitizers as increasing dose clearly indicates their expenditure in the crosslinking (Figure 1).
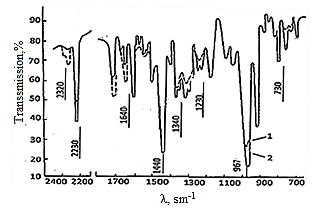 | Figure 1. IR spectra were irradiated model mixtures based Neoprene W: 1-unirradiated Neoprene W; 2- Neoprene W+ DAEMA; 3-Neoprene W + ED + DAEMA + ZnO after irradiation dosa with 500 kGy |
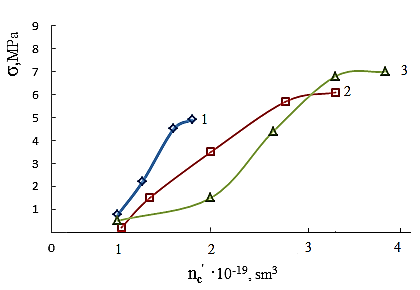
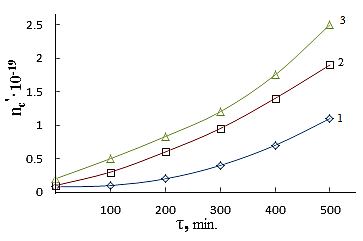 | Figure 3. The kinetics formation of output number of cross-linking 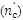 at the vulcanizing Neoprene W (D = 500 kGy): 1-Neoprene W; 2-Neoprene W + ED-5; 3-Neoprene W + DAEMA at the vulcanizing Neoprene W (D = 500 kGy): 1-Neoprene W; 2-Neoprene W + ED-5; 3-Neoprene W + DAEMA |
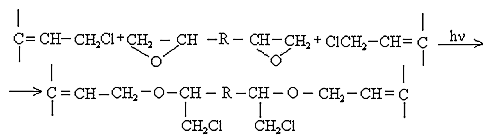 However, the effect of resin crosslinking on the process Neoprene W slightly and is at the same rate and to the same level as that involving DAEMA. Therefore provide for the participation of the double bonds of the polymer will be visible properly.Enough firmly certain action of the epoxy resin and zinc oxide on the process of emerging cross-linking was found. This can be explained by the interaction of an epoxide with zinc chloride to form compounds type-
However, the effect of resin crosslinking on the process Neoprene W slightly and is at the same rate and to the same level as that involving DAEMA. Therefore provide for the participation of the double bonds of the polymer will be visible properly.Enough firmly certain action of the epoxy resin and zinc oxide on the process of emerging cross-linking was found. This can be explained by the interaction of an epoxide with zinc chloride to form compounds type-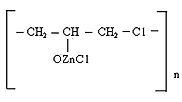 Some increase in cross-linked molecules in the presence of zinc oxide compared mixtures without oxides (Tab.2.) and close values 1 / Mnτ to settlement suggest the possibility that the presence of zinc oxide crosslinking is emptive for allylchloro, in the formation of new active centers suppressed oxides metals or products of their transformation. A good proof of this assumption is that the investigated elastomers values E, at the metal oxide vulcanization are very close.When administered in the system of metal oxides first occurs intramolecular hydrogen chloride, resulting in formation of conjugated double bonds, [2], which are then DAEMA is grafted and is sewn in the molecule, with participation of ES formed cyclically crosslinks.If this incarceration is valid, that offered low molecular substances at the interaction with the double bond, should raise the rate and degree of crosslinking.From the submitted material be seen that vulcanizates Neoprene W obtained by the action of ionizing radiation on the structure is much more complicated.One of the characteristics of features radiation vulcanization of Neoprene W is the formation of a sufficiently large number of durable C-C bonds in participation crosslinking agents.As shown by change unsaturation elastomer Neoprene W, is defined with Fourier method spectroscopy resulting in dense grids, which are compares with the mechanical properties and crystallization of vulcanizates, the value of change unsaturation value is within the accuracy of the method. The received output of the effective concentration of the cross links at 500 kGy is equal to nc' = 6 × 10-19 cm3.The use of such dosages for the comparison is justified, since the rate of change of unsaturation remains constant over a wide range of doses.Observed changes in the intensity of the absorption bands of 780 cm-1, corresponding to the double bond in the 1,4-cis configuration. Changes in these bands may be caused by the cis-trans isomerization of the elastomer, and the costs of the double bonds.For all doses the degree of crystallinity increases linearly with extension of the sample 1-4 his suspenders (Figure 4), which allows to characterize the intensity of crystal formation for given concentration slope of tangent of the angle annoy tilt of the straight abscissa.
Some increase in cross-linked molecules in the presence of zinc oxide compared mixtures without oxides (Tab.2.) and close values 1 / Mnτ to settlement suggest the possibility that the presence of zinc oxide crosslinking is emptive for allylchloro, in the formation of new active centers suppressed oxides metals or products of their transformation. A good proof of this assumption is that the investigated elastomers values E, at the metal oxide vulcanization are very close.When administered in the system of metal oxides first occurs intramolecular hydrogen chloride, resulting in formation of conjugated double bonds, [2], which are then DAEMA is grafted and is sewn in the molecule, with participation of ES formed cyclically crosslinks.If this incarceration is valid, that offered low molecular substances at the interaction with the double bond, should raise the rate and degree of crosslinking.From the submitted material be seen that vulcanizates Neoprene W obtained by the action of ionizing radiation on the structure is much more complicated.One of the characteristics of features radiation vulcanization of Neoprene W is the formation of a sufficiently large number of durable C-C bonds in participation crosslinking agents.As shown by change unsaturation elastomer Neoprene W, is defined with Fourier method spectroscopy resulting in dense grids, which are compares with the mechanical properties and crystallization of vulcanizates, the value of change unsaturation value is within the accuracy of the method. The received output of the effective concentration of the cross links at 500 kGy is equal to nc' = 6 × 10-19 cm3.The use of such dosages for the comparison is justified, since the rate of change of unsaturation remains constant over a wide range of doses.Observed changes in the intensity of the absorption bands of 780 cm-1, corresponding to the double bond in the 1,4-cis configuration. Changes in these bands may be caused by the cis-trans isomerization of the elastomer, and the costs of the double bonds.For all doses the degree of crystallinity increases linearly with extension of the sample 1-4 his suspenders (Figure 4), which allows to characterize the intensity of crystal formation for given concentration slope of tangent of the angle annoy tilt of the straight abscissa.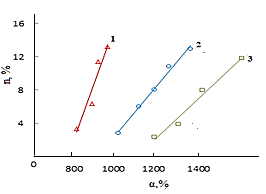 | Figure 4. The dependence of the crystallinity index of the degree of stretching for Neoprene W (1-4) irradiated at 500 kGy Direct correspond to different values 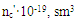 ; 1-1,8; 2-2,4; 3-3,9 ; 1-1,8; 2-2,4; 3-3,9 |
|
|
4. Conclusions
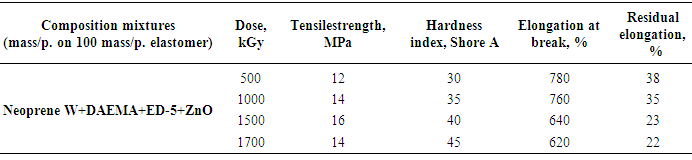
- Results of analyzes and individual properties from the structure of Neoprene W and the vulcanizing group (DAEMA, ED-5) should make two remarks. First, studies have shown that for heterogeneous radiation vulcanizates Neoprene W elastomers fundamentally observes the same regularities that have been installed in the works [2] and other researchers on the examples vulcanizates of general purpose elastomers.Second, the absence in a number of cases, the characteristics of structure of the radiation vulcanizates accounts to limit the influence on the properties type of low molecular group (DAEMA, ES). Probably, occur to the formation of intramolecular of crosslinks. Question on the reaction of epoxy resins in Neoprene W at the radiation crosslinking is much more complicated. They can disconnect hydrogen chloride and form a α-Chloroxy compound. It is also possible accession of epoxy resin to the double bond.Crosslinking metal oxide provides the highest rate of radiation-chemical yield of the number of cross-linked molecules and efficient crosslinks. The highest exposure dose leads them melting. Thus, for example, at equal concentration of crosslinks oxide vulcanizates have a fine-grained structure and interval dose of crosslinks 500-800 kGy. This is apparently due to the presence bonds of the C-C or C-O-C and a large share of movable labile bonds in the oxide vulcanizates.Between strength and concentration of crosslinks, as the expected result, there are close to a linear relationship (Figure 3).The density of the vulcanization grid, in the presence of zinc oxide, do not strongly influences on the strength of rubber. Vulcanized elastomeric mixture (W + Neoprene DAEMA + ED-5 + ZnO) is significantly different in strength from other vulcanizates. It is shown that the filling of the elastomer in the presence of technical carbon static gap strength and the Shore hardness increases with increasing irradiation dose (Table 4) at the same time decreases relative elongation. Above 1500 kGy observes a gap at the polymer mesh.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML