| [1] | A. Charlesby, “Atomic radiation and polymers,” Pergamon Press, New York, pp. 556, 1960. |
| [2] | Sh. M. Mammadov, A. A. Garibov, “Radiation physics & chemical of polymer”, LAP Lambert Ac. Publ., Germany, pp. 654, 2015. |
| [3] | J. G. Drobny, “Radiation Technology for Polymers”, CRC Press, pp. 307, 2010. |
| [4] | M. Maiti, M. Bhattacharya and A. K. Bhowmick “Elastomer Nanocomposites”, Rubber Chemistry and Technology, vol. 81, no. 3, pp. 384-469, 2008. |
| [5] | V. Vijayabaskar, M. Stephan, S. Kalaivani, S. Volke, G. Heinrich, H. Dorschner and Anil K. Bhowmick, “Influence of radiation temperature on the crosslinking of nitrile rubber by electron beam irradiation”, Radiation Physics and Chemistry, vol. 77, no. 4, pp. 511-521, 2008. |
| [6] | R. J. Woods and A. K. Pikayev, “Applied radiation chemistry: radiation processing”, John Wiley & Sons, pp. 535, 1994. |
| [7] | Sh. M. Mammadov, “Synthesis, processing and vulcanization of NBR”, LAP. Lambert Academic, Germany, pp. 340, 2015. |
| [8] | A. D. Pomogaylo, A.S. Rosenberg, I. E. Uflyand, “Nanoparticles of metals in polymers”, Khimiya, Moscow, pp. 672, 2002. |
| [9] | C. N. R. Rao, A. Müller, A. K. Cheetham (Eds.) “The Chemistry of Nanomaterials Synthesis, Properties and Applications”, vol. 2, John Wiley & Sons, 761 pp., 2004. |
| [10] | M. Akay, “İntroduction to polymer science and technology”, MA & Ventus Publishing ApS, pp.269, 2012. |
| [11] | J. R. Dunn, 'Carboxylated Rubber' in "Handbook of Elastomers", 2nd ed., A. K.. Bowmick, and H. L. Stephens, Eds., Marcel Dekker, New York, pp. 561, 2001. |
| [12] | E. V. Kuznetsov, S. M. Divgun, L. A. Budarina “Praktikum po khimii I fiziki polimerov” (Laboratory Works on Polymer Chemistry and Physics), Moscow, Khimiya, pp.380, 1977. |
| [13] | P. J. Flory, J. Rehner, “Statistical mechanics of crosslinked polymer networks. II. Swelling” Journal of Chemical Physics, vol. 11, pp. 521–526, 1943. |
| [14] | L. J. Bellamy, “The infrared spectra of complex molecules”, vol. 2, 2nd ed., Chaman and Hall, London, 299 pp. 1980. |
| [15] | N. Q. Bakhshiyev, “Spektroskopiya mejmolekulyarnıkh vzaimodeystviy”, Sank-Peterburg. Nauka, pp.328, 1982 |
| [16] | M. Litvinov, P. De. Prajna, “Spectroscopy of Rubbers and Rubbery Materials”, iSmithers Rapra Pub., pp. 638, 2002 |
| [17] | O. V. Krylov, “Catalysis by Non-Metals”, Academic New York, pp.131, 1970. |
| [18] | S. Sahoo, M. Maiti, A. Ganguly, J. Jacob George, A.K. Bhowmick, “Effect of zinc oxide nanoparticles as cure activator on the properties of natural rubber and nitrile rubber”, Journal of Applied Polymer Science, vol. 105, no.4, pp. 2407-2415, 2007. |
| [19] | Sh. M. Mammadov, S. V. Rzaeva, R. H. Mehdiyeva, H. N. Akhundzada, E. N. Ahmadov, “Structural Parameters and Dynamical Properties of the Radiative and Peroxide Vulcanizates Based on EPM Participation”, American Journal of Polymer Science, vol. 6, no.1, pp. 12-17, 2016. |
| [20] | T. S. Gates and J. A. Hinkley, “Computational Materials: Modeling and Simulation of Nanostructure Materials and Systems”, NASA/TM- 2003-212163, Mar., pp. 1– 17, 2003. |
| [21] | G. Heideman, J. W. M. Noordermeer, R. N. Datta and B. Baarle, “Activators in accelerated sulfur vulcanization”, RUB. CHEM. AND TECHNOL, vol. 77, no. 3, pp. 512-541, 2005. |
| [22] | M. Przybyszewska, M. Zaborski, “The effect of zinc oxide nanoparticle morphology on activity in crosslinking of carboxylated nitrile elastomer”, eXPRESS Polymer Letters vol.3, no.9, pp. 542–552, 2009. |
| [23] | Sh. M. Mammadov, A. A. Garibov, J. S. Mammadov, “Interactions of Sensitizer Disulfochloride Aromatic Compounds with Unsaturated Elastomers” International Journal of Advanced Chemical Technology, vol. 2, no.3 2013. |
| [24] | Sh. M. Mammadov, S. A Rzayeva, A. А. Garibov, R. N. Mehdiyeva, J. S. Mammadov, “Radiation-Chemical Structure of Acrylo-Nitrile Butadiene Rubber with Copolymer Vinyl Chloride and Vinyl Acetate”, American Journal of Polymer Science, vol. 3, no.4, pp. 76-82, 2013. |
| [25] | Sh. M. Mammadov, A. А. Garibov, О. A. Аkparov, А. K. Salehov, S. S. Ahmadova, “The Influence of γ-Irradiation on Structuration in Solutions of BNR and Properties of Films”, Open Journal of Physical Chemistry, vol. 2, pp. 182-184, 2012. |
| [26] | Sh. M. Mammadov, A.A. Garibov, “Radiolysis of butadiene-nitrile blend with polyvinylchloride”, Chemistry of High Energy, vol.44, no.4, pp. 298-301, 2010. |
| [27] | Sh. M. Mammadov, R. F. Khankisiyeva, J. S. Mammadov “The Influence of Irradiation and Temperature to the Nature of Crosslinking in isoprene nitrile elastomer, Universal Journal of Chemistry, vol.3, no.2, pp. 49-54, 2015. |
| [28] | K. J. İvin, “Structural Studies of Macromolecules by Spectroscopic Methods”, J. Wiley, pp.76, 1980. |
| [29] | D. I. Bower and W. F. Maddams, “The vibrational spectroscopy of Polymer”, Cambridge University. Press, New York, pp. 326, 1992. |
| [30] | H. Mou, F. Shen, Q. Shi, Y. Liu, C. Wu and W. Guo, A novel nitrile butadiene rubber/zinc chloride composite: Coordination reaction and miscibility, European Polymer Journal, vol.48, no. 4, 2012. |
| [31] | M. Przybyszewska, M. Zaborski, “Nanoparticle zinc oxide applied for crosslinking of butadiene rubbers” in ‘8th European Symposium on Polymer Blends and Eurofillers. Bruges, Belgium’, pp. 116, 2005. |
| [32] | H. Althues, P. Simon, F. Philipp and S. Kaskel, İntegration of zinc oxide nanoparticles into transparent poly (butanediol-monoacrylate) via photopolymerisation, Journal of Nanoscience and Nanotechnology, Vol. 6 (2), 409-413 (2006). |
| [33] | E. Elshereafy, M. A. Mohamed, M. M. Zayat, A.A. Miligy “Gamma radiation curing of nitrile rubber/high density polyethylene blends”, Radiation Chemistry, Radiocehemistry And Nuclear Chemistry, vol.293, no.3, pp. 941-947, 2012. |


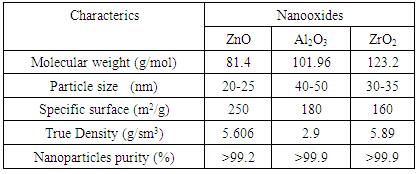
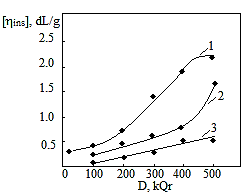
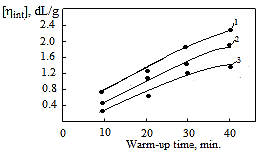
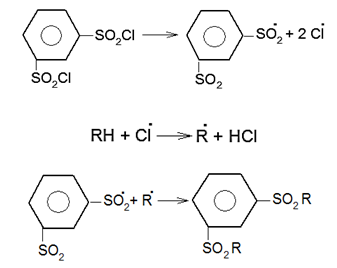
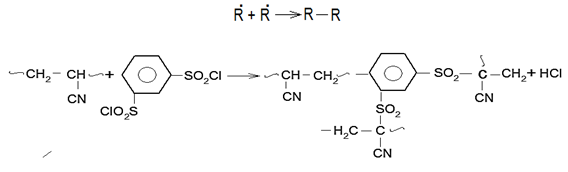
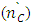 is not filled in the thermal radiation and thermo-radiation vulcanizates, which was calculated from the results obtained by the equilibrium swelling in toluene [12]. The number of crosslinked molecules
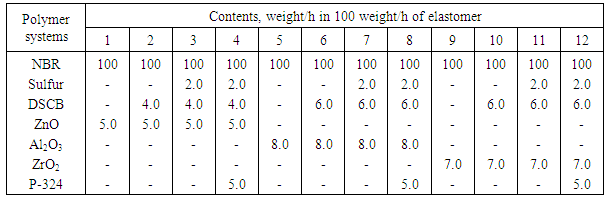
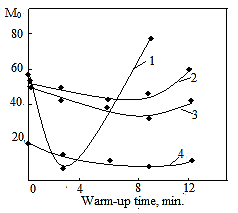
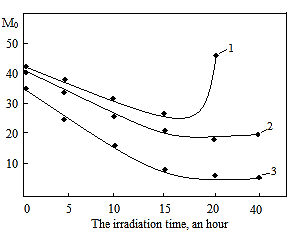
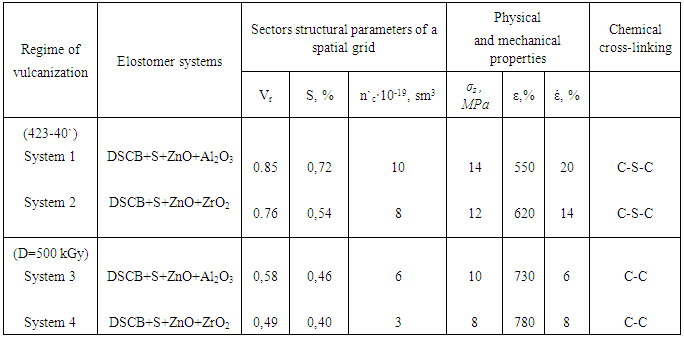
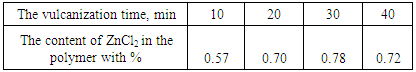
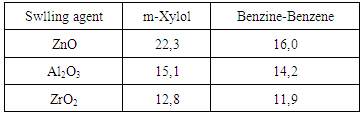
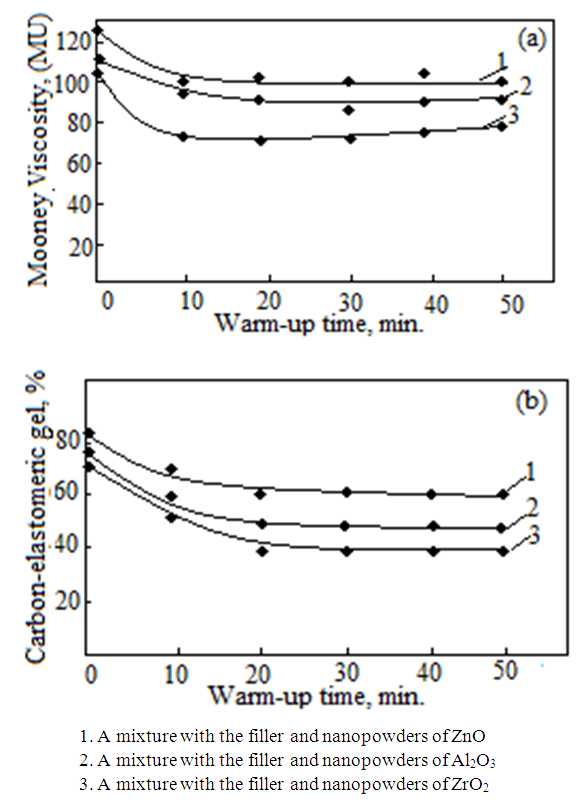
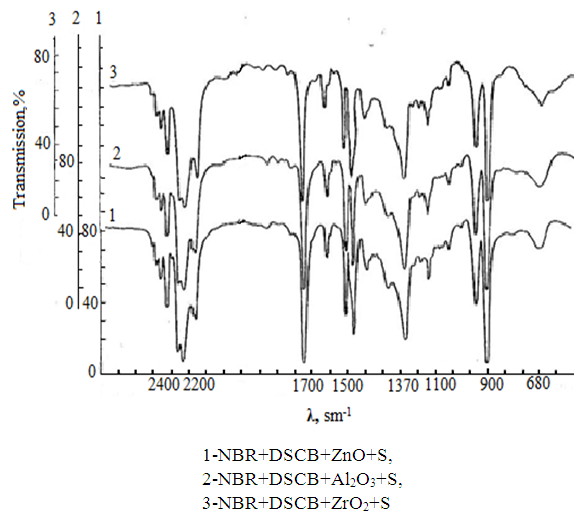
is not filled in the thermal radiation and thermo-radiation vulcanizates, which was calculated from the results obtained by the equilibrium swelling in toluene [12]. The number of crosslinked molecules 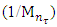 was determined by a sol-gel analysis. Calculation of the spatial grid parameters of thermal, radiation samples were determined according to the formula of Flory-Rehner [13]. The change of the molecular structure of cross-linked elastomer was determined by the method of Fourier spectroscopy. Identification of the spectra was performed according to the correlation table. [14-16]. Assessment of the impact of nano-sized metal oxide powder, crosslinking additives and carbon black in the mechanical and dynamic properties were performed on the following parameters: tensile strength, elongation after fracture, stress relaxation and conditional equilibrium modulus.Rheological plasticity and nanocomposites were determined on a Mooney viscometer (VM-2004 firm Cibitre Sri-Instruments) and PSM-2 Rheometer (Germany).
was determined by a sol-gel analysis. Calculation of the spatial grid parameters of thermal, radiation samples were determined according to the formula of Flory-Rehner [13]. The change of the molecular structure of cross-linked elastomer was determined by the method of Fourier spectroscopy. Identification of the spectra was performed according to the correlation table. [14-16]. Assessment of the impact of nano-sized metal oxide powder, crosslinking additives and carbon black in the mechanical and dynamic properties were performed on the following parameters: tensile strength, elongation after fracture, stress relaxation and conditional equilibrium modulus.Rheological plasticity and nanocomposites were determined on a Mooney viscometer (VM-2004 firm Cibitre Sri-Instruments) and PSM-2 Rheometer (Germany).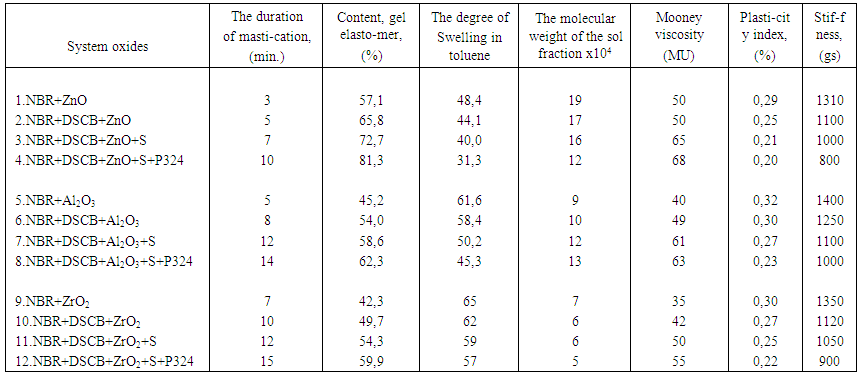
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML