| [1] | Ganji F, Abdekhodaie MJ, Ramazany-Sadtabadi A, Gelation time and degradation rate of chitosan as a thermosensitive injectable hydrogel, J Sol-Gel Sci Technol, 2007, 42, 47-53. |
| [2] | Ganji F, Abdekhodaie MJ, Synthesis and charac- terization of a new thermoreversible chitosan-PEG diblock copolymer, Carbohydr Polym, 2008,74, 435-441. |
| [3] | Hosseinkhani H, Hosseinkhani M, Khademhosseini A, Enhanced angiogenesis through controlled release of basic fibroblast growth factor from peptide amphiphile for tissue regeneration, Biomaterials, 2006, 27, 5836-5844. |
| [4] | CM Hassen, NA Peppas, Adv. Polym. Sci., 2000 153, 37. |
| [5] | Syed Majid Hanif Bukhari, Samiullah Khan, Muhammad Rehanullah, and Nazar Mohammad Ranjha, Synthesis and Characterization of Chemically Cross-Linked Acrylic Acid/Gelatin Hydrogels: Effect of pH and Composition on Swelling and Drug Release, International Journal of Polymer Science, 2015 (2015), Article ID 187961, 15. |
| [6] | K Kamth, K Park, Adv. Drug. Deliv. Rev, 1993, 11, 59. |
| [7] | R Langer, Chem. Eng. Commun., 1990, 6, 1. |
| [8] | AJ Aleyamma and CP Sharma, In Blood Compatiable Materials and Devices-Perspective towards the 21st Century. Sharma CP and Szycher M Eds., Technomic, Lancaster, PA, 1991, p. 123. |
| [9] | Vinod Kumar G S, B Mathew, Eur. Polym. Mater., 1998, 34, 1185. |
| [10] | X Li, Y Huang, J Xiao, C Yan, J. Appl. Polym. Sci., 1995, 55,1779. |
| [11] | M Andersson, A Axelsson , G Zacchi, J. Controlled. Release., 1998, 50,273. |
| [12] | F Eeckman, AJ Moes, K Amighi, Eur. Polym. J., 2004, 40, 873. |
| [13] | H Kasgoz, S Ozgumu, M Orbay, Polymer, 2001, 42,7497. |
| [14] | D Saraydin, E Karadag, N Oztop, O Guven, Biomaterials, 1994, 5, 917. |
| [15] | E Karadag, D Saraydin, N Oztop, O Guven, Polym. Adv. Technol, 1994, 5, 664. |
| [16] | S Donempudi, MJ Yaseen, Polym. Mater., 1994,11,73. |
| [17] | NA Peppas, DJ Am Ede, J. Appl. Polym. Sci, 1997, 65, 509. |
| [18] | Ashish kumar gupta, Satyawan Singh, SN Pandeya, Aparna Misra, Abhishek Gupta, Kiran gupta, M. Bajpai, Der pharmacia, 2010, 2, 6, 68-75. |
| [19] | Yin Y, Yang Y, Xu H, Swelling behavior of hydrogels for colon-site drug delivery, J Appl Polym Sci., 2002, 83, 2835-2842. |
| [20] | Bakhshi R, Vasheghani-Farahani E, Mobedi H, Jamshidi A, Khakpour M, The effect of additives on naltrexone hydrochloride release and solvent removal rate from an injectable in situ forming PLGA implant, Polym Adv Technol, 2006,17, 341-346. |
| [21] | Tavakol M, Vasheghani-Farahani E, Dolatabadi- Farahani T, Hashemi-Najafabadi S, Sulfasalazine release from alginate-N,O-carboxymethyl chitosan gel beads coated by chitosan, Carbohydr Polym, 2009, 77, 326-330. |
| [22] | I. C. Kwon, Y.H. Bae, S.W. Kim, Electrically erodible poly- Biophys. J. 9, 1969, 700–728. |
| [23] | I. C. Kwon, Y.H. Bae, S.W. Kim, Heparin release from perimental, Biophys. J. 9, 1969, 729–757. |
| [24] | You Kok Yeow, Zulkifly Abbas, Kaida Khalid and Mohamad Zaki Abdul Rahman. Improved Dielectric Model for Polyvinyl Alcohol-Water Hydrogel at Microwave Frequencies, American. Journal of Applied Sciences, 2010,7 (2): 270-276. |
| [25] | Abd El-kader, F. H.; Osman, W. H; Mahmoud, K. H. and Basha, M. A. F. “Dielectric investigations and ac conductivity of polyvinyl alcohol films doped with europium and terbium chloride”. Physica B: Condensed Matter, 2008, vol. 403, No. 19-20 (October), pp. 3473-3484. |
| [26] | Awadhia, A.; Patel, S. K. and Agrawal, S. L. “Dielectric investigations in PVA based gel electrolytes”. Progress in Crystal Growth and Characterization of Materials, vol. 52, No. 1-2 (March - June), 2006. pp. 61-68. |
| [27] | Blyte, T. and Bloor, D. Electrical properties of polymers. Cambridge, UK: Cambridge University Press, 2005. 480 |
| [28] | Casalini, R. and Roland, C. M. “Effect of crosslinking on the secondary relaxation in polyvinylethylene”. Journal of Polymer Science. Part B: Polymer Physics, 2010, vol. 48, No. 5, pp. 582-587. |
| [29] | P. K. C. Pillai, G. K. Narula and A. K. Tripathi, Polym. J., 1984, 16,575. |
| [30] | Leyla Aras and Bahattin M. Baysal, J. Polym. Sci. Polym. Phys. Edn., 1984, 22, 1453. |
| [31] | Parimal Maji, Arijit Gandhi, Sougata Jana, & Nirmal Maji, “Preparation and characterization of maleic anhydride cross-Linked chitosan-polyvinyl alcohol hydrogel matrix transdermal patch”, Journal of PharmaSciTech, 2013, 2(2): 62-67. |
| [32] | T. Jamnongkan, & S. Kaewpirom, “Controlled-Release Fertilizer Based on Chitosan Hydrogel: Phosphorus Release Kinetics”, Sci. J. UBU, 2010,Vol. 1, No. 1, 43-50. |
| [33] | Pritam Roy and Raj Kumar Dutta, “Electrospun PVA-Pectin-Magnetite nanofiber as a novel drug carrier matrix”, International Journal of Applied Engineering Research , 2014, Volume 9, Number 6, pp. 629-635. |
| [34] | A. K. Bajpai, J. Bajpai, & S. N. Soni, “Preparation and characterization of electrically conductive composites of poly (vinyl alcohol)-g-poly (acrylic acid) hydrogels impregnated with polyaniline (PANI)”, eXPRESS Polymer Letters, 2008,Vol.2, No.1, 26–39. |
| [35] | Ali Pourjavadi, & Gholam Reza Mahdavinia, “Superabsorbency, pH-sensitivity and swelling kinetics of partially hydrolyzed chitosan-g-poly(acrylamide) hydrogels”, 2006, Turk J. Chem., 30, 595 – 608. |
| [36] | Michel Bocourt Povea, Waldo Argüelles Monal, Juan Valerio Cauich Rodríguez, Alejando May Pat, Nancy Badas Rivero, & Carlos Peniche Covas, “Interpenetrated chitosan-poly (acrylic acid-Co-acrylamide) hydrogels. synthesis, characterization and sustained protein release studies”, Materials Sciences and Applications, 2011, 2, 509-520. |
| [37] | M. Efentakis, M. Vlachou, & N. H. Choulis, “Effects of excipients on swelling and drug release from compressed matrices”, Drug Development and Industrial Pharmacy, 1997, 23(1), 107 - 112. |
| [38] | Seyfullah Madakbas, Kadir Esmer, Ersin Kayahan, & Mehmet Yumak, “Synthesis and Dielectric Properties of Poly(aniline)/Na-bentonite Nanocomposite”, Science and Engineering of Composite Materials, 2010, 17, 145-153. |
| [39] | A. K. Bajpai, J. Bajpai, & S. N. Soni, “Preparation and characterization of electrically conductive composites of poly (vinyl alcohol)-g-poly(acrylic acid) hydrogels impregnated with polyaniline (PANI)”, eXPRESS Polymer Letters , 2008, Vol.2, No.1, 26–39. |
| [40] | Ali Pourjavadi, & Gholam Reza Mahdavinia, “Superabsorbency, pH-sensitivity and swelling kinetics of partially hydrolyzed chitosan-g-poly(acrylamide) hydrogels”, Turk J. Chem., 2006, 30, 595 – 608. |
| [41] | Michel Bocourt Povea, Waldo Argüelles Monal, Juan Valerio Cauich Rodríguez, Alejando May Pat, Nancy Badas Rivero, & Carlos Peniche Covas, “Interpenetrated chitosan-poly (acrylic acid-Co-acrylamide) hydrogels. synthesis, characterization and sustained protein release studies”, Materials Sciences and Applications, 2011, 2, 509-520. |
| [42] | Pawlak, A. and Mucha, M., “Thermogravimetric and FTIR studies of chitosan blends”, Thermochimica Acta., 2003,396: 153-166. |
| [43] | Zheng, H., Du, Y.M., Yu, J.H., Huang, R.H. and Zhang, L.N., “Preparation and charecterization of chitson/polyvinyl alcohol blend fibres”, J. Appl. Polym. Sci., 2001, 80(13): 2558–2565. |
| [44] | Keno, H.Y., Um, I.C. and Park, Y.H., “Structural and thermal characteristics of Antheraea pernyi silk fibroin/chitosan blend film”, Polymer , 2001,42: 6651-6656. |
| [45] | Sinitsya, A., Copikova, J., Matejka, P. and Machovic, V., Carbohy. Polym., 2003,54: 97 . |
| [46] | Coimbra, M.A., Barros, A., Barros, M., Rutledge, D.N. and Delgadillo, I., Carbohy. Polym.,1998, 37: 241. |
| [47] | Wang T, Turhan M, & Gunasekaran S., “Selected properties of pH-sensitive, biodegradable chitosan-poly(vinyl alcohol) hydrogel”, Polym Int., 2004, 53(7):911–918. |
| [48] | Mansur HS, Sadahira CM, Souza AN, & Mansur AAP, “FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde”, Mater Sci Eng C., 2008, 28(4): 539–548. |
| [49] | P. Rama Subba Reddy, S. Eswaramma, K. S. V. Krishna Rao, and Yong Ill Lee, “Dual Responsive Pectin Hydrogels and Their Silver Nanocomposites: Swelling Studies, Controlled Drug Delivery and Antimicrobial Applications”, Bull. Korean Chem. Soc., 2014, Vol. 35, No. 8, 2391. |
| [50] | U. Edlund, and A. C. Albertsson, “Degradable Polymer Microspheres for Controlled Drug Delivery,” Advances in Polymer Science, 2002, Vol. 157, pp. 67-112. |
| [51] | D. Sahoo, S. Sahoo, J. Das, T. K. Dangar and P. L. Na- yak, “Antibacterial Activity of Chitosan Crosslinked with Aldehydes and Blended with Cloisite 30B,” NanoTrends , 2011, Vol. 10, pp. 1-9. |
| [52] | J. M. Gohil, A. Bhattacharya and P. Ray, “Studies on the cross-linking of poly (vinyl alcohol)”, Journal of Polymer Research, 2006, 13,161-169. |
| [53] | Long Xu, Yun-An Huang, Qiu-Jin Zhu, and Chun Ye, “Chitosan in Molecularly-Imprinted Polymers: Current and Future Prospects”, Int. J. Mol. Sci. , 2015,16, 18328-18347. |
| [54] | Dai WS, Barbari TA. Hydrogel membranes with mesh size asymmetry based on the gradient crosslinking of poly (vinyl alcohol). J Membr Sci 1999, 156, 67–79. |
| [55] | Peppas NA, Berner Jr RE Proposed method of intracopdal injection and gelation of poly (vinyl alcohol) solution in vocal cords: polymer considerations. Biomaterials, 1, 1980, 158–162. |
| [56] | C. C. Ku, R. Liepins, “Electrical Properties of Polymers”, Hanserp, 20–58. New York 1987. |
| [57] | A. Patsidis, G. C. Psarras. “Dielectric behaviour and functionality of polymer matrix–ceramic BaTiO3 composites”. eXPRESS Polymer Letters 2, 2008, no.10 718–726. |
| [58] | I. Latif, E. E. AL-Abodi, D. H. Badri, J. Al Khafagi, “Preparation, Characterization and Electrical Study of (Carboxymethylated Polyvinyl Alcohol/ZnO) Nanocomposites”, American Journal of Polymer Science, 2012, 2(6): 135-140. |
| [59] | I. Latif, T. B. Alwan, A. H. Al-Dujaili, “Low Frequency Dielectric Study of PAPA-PVA-GR Nanocomposites”, Nanoscience and Nanotechnology, 2012, 2(6): 190-200. |
| [60] | I. A. LATIF1, H. M. ABDULLAH, S. H. MARZA, A. H. AL-DUJAILI and E. T. BAKIR, “Synthesis, Characterization and Electrical Properties of ZnO Nanoparticles Dispersed in Poly(vinyl acetal)/PVA Composite”, Asian Journal of Chemistry, 2013, 25, 12 , 7753-7757. |
| [61] | G. M.Tsangaris, G. C. Psarras, N. Kouloumbi, "Polyurethane latex/water dispersible boehmite alumina nanocomposites: “Thermal, mechanical and dielectrical properties", RSC Publisher”, Journal of Materials Science, 1998, 33(8), 2027-2037. |
| [62] | K. G. Gatos, A. J. G. Martínez, G. C. Psarras, R. T. Karger-Kocsis, J Elsevier, “Composites Science and Technology”, 2007, 67(2), 157-167. |
| [63] | P G. C. sarras, K. G. Gatos, P. K. Karahaliou, S. N. Georga, Krontiras CA, Karger-Kocsis J "Relaxation phenomena in rubber/ layered silicate nanocomposites", Springer, Express Polymer Letters, 1, 2007, 837-845. |



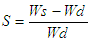
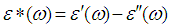
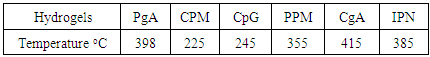
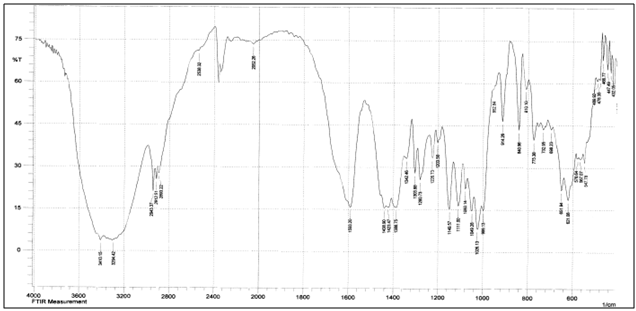
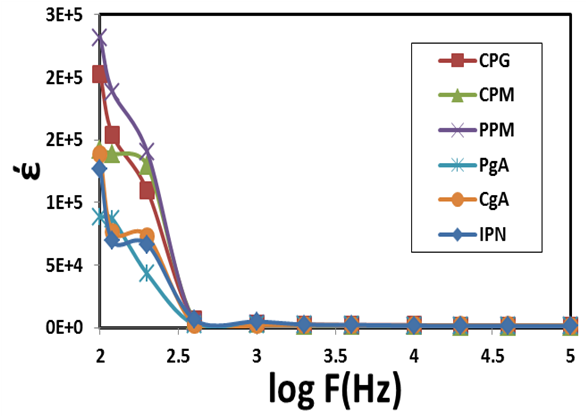
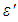 and
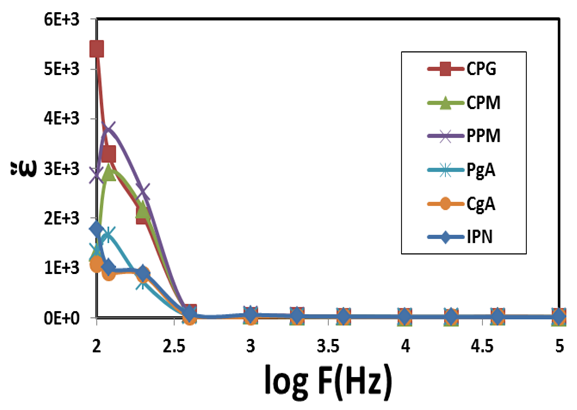
and 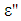 were the real part and imaginary part components of the energy storage and loss, respectively, in each cycle of the electrical field. The measured capacitance C was used to calculate the dielectric constant
were the real part and imaginary part components of the energy storage and loss, respectively, in each cycle of the electrical field. The measured capacitance C was used to calculate the dielectric constant 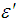 , using the following expression,
, using the following expression,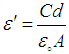
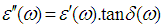
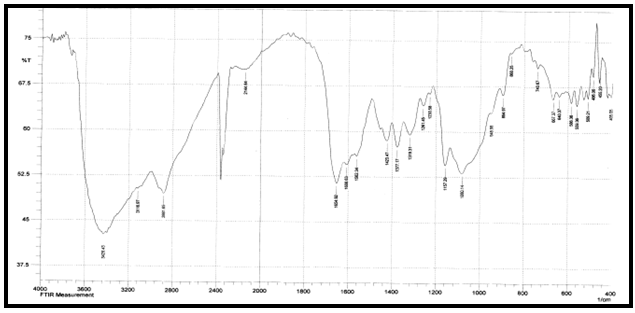
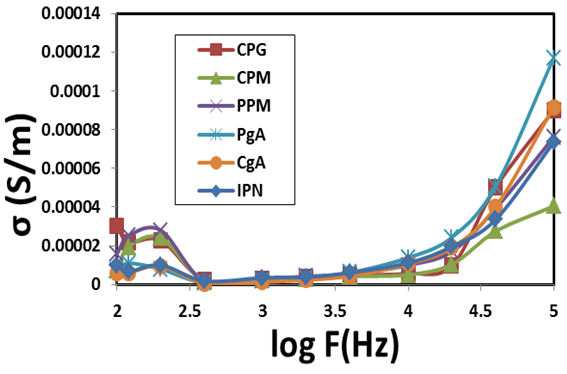
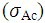 calculated by the following equation [5].
calculated by the following equation [5]. 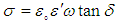
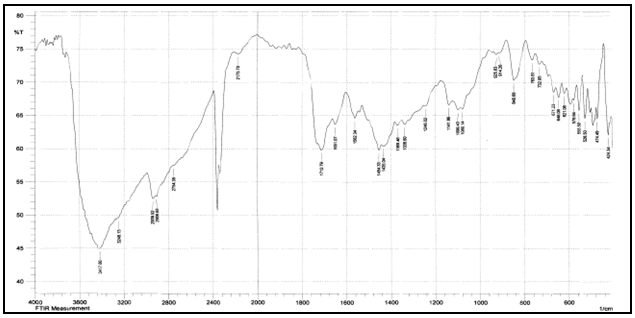
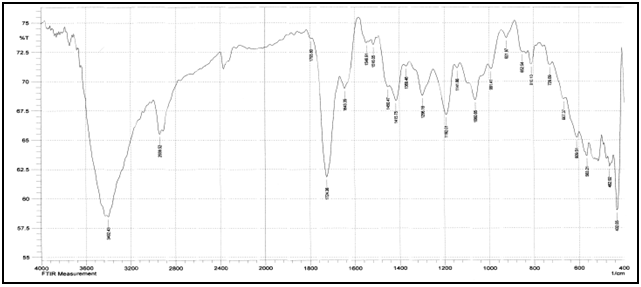
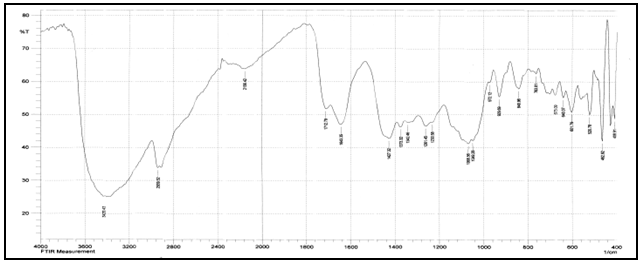
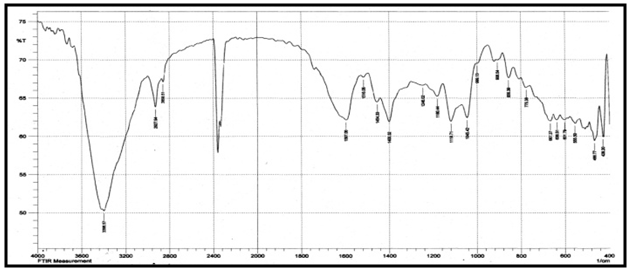
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML