-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Polymer Science
p-ISSN: 2163-1344 e-ISSN: 2163-1352
2016; 6(2): 46-49
doi:10.5923/j.ajps.20160602.03

Study Sorption of Heavy Metals Nitrogen – And - Phosphorus Containing Polyampholytes
D. J. Bekchanov , N. J. Sagdiev , M. G. Mukhamediev
Department of Chemistry, National University of Uzbekistan Named after Mirzo Ulugbek, Tashkent, Uzbekistan
Correspondence to: N. J. Sagdiev , Department of Chemistry, National University of Uzbekistan Named after Mirzo Ulugbek, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

We have investigated the sorption copper (II), nickel (II) and indium (III) ions on a phosphorus based polyampholytes granular polyvinylchloride. We have established the dependence of static adsorption from aqueous solutions with metal ion concentration and temperature of the system. Isotherms were constructed and found the adsorption equilibrium constants, the thermodynamic parameters of the process - isobaric - isothermal potential (ΔG), enthalpy (ΔH) and entropy (ΔS). It is shown that the investigated sorbent absorbs ions Cu (II) to a greater extent than the other ions.
Keywords: Nitrogen and phosphorous polyampholyte, Sorption, Ions of copper, Indium and nickel, Chemisorption and thermodynamic parameters
Cite this paper: D. J. Bekchanov , N. J. Sagdiev , M. G. Mukhamediev , Study Sorption of Heavy Metals Nitrogen – And - Phosphorus Containing Polyampholytes, American Journal of Polymer Science, Vol. 6 No. 2, 2016, pp. 46-49. doi: 10.5923/j.ajps.20160602.03.
1. Introduction
- To solve the technological problems associated with obtaining high-purity substances in separation processes, extraction and concentration of rare and non-ferrous metals should be used chelating resins having a greater affinity for the studied ions. Such sorbents include nitrogen and phosphorus polyampholytes having in its composition and amino phosphonic group [1-4]. Such sequestering polymers may be used in non-ferrous metallurgy, hydrometallurgy for recovery of indium, copper and nickel from waste water in the chemical industry, and also for producing substances of various natures.Complexing sorbents useful for the selective adsorption of indium can be divided into two classes: the sorbents containing iminodiacetic groups and sorbents containing phosphonic groups [5-7].A serious drawback of the extraction of indium carriers containing iminodiacetic groups are is need for sorption at pH «1.5 at which these sorbents have the greatest selectivity for indium, which significantly complicates the technology of extracting indium from real technological solutions [5]. Similarly to the significant disadvantages of this class include low capacity adsorbents with respect to indium (0.184 mmol /g) in the conditions of maximum selectivity (70-80%).Sorbents second class containing phosphonic groups can be divided into carriers having in its composition aminomethylphosphonic, monophosphonic and gemdiphosphonic groups [6].The advantages of the second class of sorbents can be attributed to their relatively high capacity and selectivity to indium in the less acidic solutions (pH ~ 4). This fact is most pronounced for gemdiphosphonic sorbents containing functional groups, such as carriers Diphonix Resin (Eichrom Industries) obtained by copolymerization of acrylonitrile, divinylbenzene, styrene and phosphorus containing monomer, followed by hydrolysis and sulfonation of the resulting product. The disadvantages of this method are: a reduced selectivity for indium, the complexity and multi-stage production process, the long duration of the production cycle, the use of expensive and not readily available phosphorus-containing monomer required for the introduction of gemdifosfonovyh groups [7].Therefore, in [8] offered a method for producing a complexing sorbent for the selective extraction of indium, having in its composition gemdiphosphonic functional groups. Introduction of the gemdiphosphonic functional groups is accomplished by treating the spherical crosslinked macroporous copolymer of acrylonitrile and divinylbenzene with concentrated phosphoric acid at a temperature 140-160°C within 13-35 hours. The same process, in the presence of a diluent (chlorobenzene), carried out at a temperature 100-130°C. The maximum value for S indium ions (III) when removed from the concentrated solution of indium sulphate (7.489 g/l) was 2.9 mg - equivalent /g. The selectivity of the sorbent in the presence of indium ions in the accompanying technological solutions was 90%.To extract ions nickel (II) used as phosphorus-containing resins. The authors [9] for the sorption of nickel (II) ion exchanger was used, based on the polymer industry - butadiene rubber stamp SKD. The ion exchanger obtained by the reaction of oxidative chlorophosphorylation ACS under the PCl3 in the presence of oxygen, followed by hydrolysis of the resulting product. The laws of kinetics of sorption of cobalt and nickel on a phosphorus-containing cations is investigated. The results obtained revealed that the rate-limiting step in the process is both external and internal diffusion. At the same time, a contribution to the overall speed of the process of making and stage interaction sorbed ions with functional groups of the cation exchanger. This SEC cation exchanger of ions nickel (II) is very small and is 0.5 mmol/g. This low capacity due to the absence of likely complexing groups in the cation resin.The paper [10] presents the results of studying the process of sorption of nickel and cobalt ion exchanger based on hydrolyzed polyacrylonitrile (GIPAN) and epichlorohydrin. The influence of the conditions for obtaining the resin: temperature of reaction, the ratio of the starting materials and solvent nature - the process of sorption of these ions is investigated. It is shown that in the process modification GIPAN epichlorohydrin formed polyampholyte complexing with ammonium and carboxyl groups in the side chain. The presence of complexing groups results in a higher value compared with the CDE and SKD it is around 1.8 meq/g.The authors [13] studied the ion exchange process for the exchange of ions of heavy metals (Cu2+, Ni2 +, Cd2+, Zn2+, and Co2+) and the ions Ca2+, Na+, and NH4+ with two commercially available ion exchange chelating resins aminophosphate functional groups (Purolite S 940 and S 950). Evaluation of the results of sorption by surface complexation theory led to a set of binary equilibrium parameters that remain the same in multicomponent systems. With the analysis of the kinetic parameters of the secondary, tertiary and quaternary equilibrium and balance of complex agents in the wastewater produced in the processing of metal surfactants have been proposed based on equations that calculated theoretical values of sorption and compared with experimental data. In all cases it was found significant matching.In [14], the authors conducted a study on the extraction of trace amounts of heavy metals by ion exchange. Experiments were made using ion exchange resin Lewatit CNP 80 (weakly acidic) and Lewatit TP 207 (slightly acidic and chelate). It is investigated the effect of pH, time, metal concentration and the amount of the ion exchanger in the sorption process. Studied chelate media showed a high and more rapid sorption capacity for metal ions such as ions of lead (II), copper (II), zinc (II), Cd (II) and Ni (II). The optimum pH range for the ion exchange of the above metal ions on Lewatit CNP 80 and Lewatit TP 207 were 7.0-9.0 and 4.5-5.5, respectively.The data show that the nitrogen and phosphorus containing ion exchangers is selective with respect to ions Cu (II), Ni (II) and In (III) to form stable complexes with them. Therefore, the present work is devoted to the analysis of sorption of Cu (II), In (III) and Ni (II) from aqueous solutions of nitrogen and phosphorus containing polyampholytes PPE-1-P.
2. Experimental Part
- Working solutions of indium nitrate and nickel nitrate was prepared by dissolving the sample Cu(NO3)2, In(NO3)3 and Ni(NO3)2 *6H2O a certain amount of distilled water. The pH of the solution was adjusted using ammonium acetate buffer solution.Sorption of copper, indium and nickel were studied by static method. For this purpose, three conical flasks was placed 0.2 g ion exchange materials PPE-1-P is poured separately nitrates solutions of copper, indium and nickel. We study the dependence of the adsorption of metal ions on the time, temperature and concentration of solutions. The concentration of ions In (III) in solution was measured by conductivity meter CAL-1M2, pH of the solution was measured using a universal ionomer EV-74 and pH-meter pH / mV / TEMP Meter P25 EcoMet. The change in concentration of ions Cu (II), and Ni (II) in solution was measured by the spectrophotometric method. The change in optical density of the solution was measured with a microplate reader Enspire Perkin Elmer (USA).Ion exchanger material PPE-1-P synthesized by modification of granular polyvinyl chloride (PVC) with amines to further processing with phosphorous acid in the presence of formaldehyde [11], in static conditions. It have cation exchange phosphonic and anion exchange amine groups.
3. Discussion of Results
- PE-1-P ion exchange material contains in its composition phosphonic group therefore studied metal cations sorption occurs due to the electrostatic binding of these metal ions of these functional groups. The presence of amino groups in the resin composition also promotes the binding of metal ions due to chelating. The degree of binding of ions depends on the degree of ionization of the functional groups of the ion exchange material, and that in turn depends on the pH. Therefore, according to a study on the sorption of the studied metal ions were carried out on the pH media.
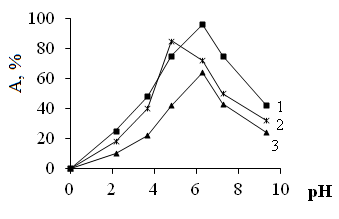 | Figure 1. Relative sorption of Cu2+, (1) Ni2+ (2) and In3+ (3) on dependence of the pH |
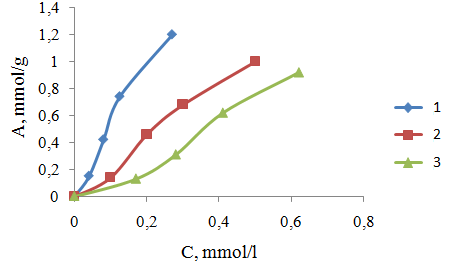 | Figure 2. Adsorption isotherms of Cu2+ ions with an ion exchanger material PPE-1-P at different temperatures. 1, 2, 3 temperature sorption 313, 303, 293oK, respectively |
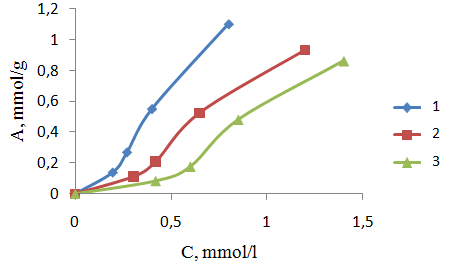 | Figure 3. Adsorption isotherms ions Ni+ 2 with PPE-1-P ion exchanger at different temperatures. 1, 2, 3 temperature sorption 313, 303, 293 oK, respectively |
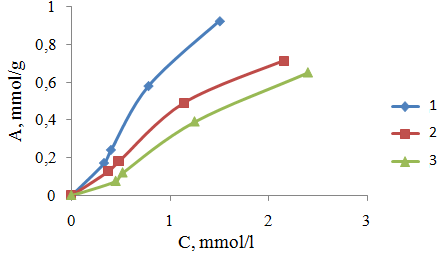 | Figure 4. The isotherms of adsorption of ions In+3 by PPE-1-P ion exchanger at different temperatures. 1, 2, 3 temperature sorption 313, 303, 293oK, respectively |
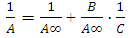 B = 1 / K. Plotted 1 /A, 1 / C, the slope of the straight line gives the value of B/A∞ and intercept on the y-axis value of 1 /A∞. Fig. 3 shows the dependence of 1 / T 1 / C process for sorption of copper ions (II) nickel (II) and indium (III) sorbent at various temperatures. We see that this dependence is a straight character, indicating the submission of the sorption theory of Langmuir monolayer,Thermodynamic functions were identified by us from the dependence of equilibrium constants on the temperature. Based on the fact that
B = 1 / K. Plotted 1 /A, 1 / C, the slope of the straight line gives the value of B/A∞ and intercept on the y-axis value of 1 /A∞. Fig. 3 shows the dependence of 1 / T 1 / C process for sorption of copper ions (II) nickel (II) and indium (III) sorbent at various temperatures. We see that this dependence is a straight character, indicating the submission of the sorption theory of Langmuir monolayer,Thermodynamic functions were identified by us from the dependence of equilibrium constants on the temperature. Based on the fact that 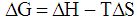 the values found ΔН and ΔS.For this RlnK plotted versus 1 / T, the slope of this line is calculated ΔН, ΔS calculated from equation
the values found ΔН and ΔS.For this RlnK plotted versus 1 / T, the slope of this line is calculated ΔН, ΔS calculated from equation 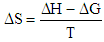 [12].The calculation results are shown in Table.
[12].The calculation results are shown in Table.
|
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML