-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Polymer Science
p-ISSN: 2163-1344 e-ISSN: 2163-1352
2016; 6(1): 18-27
doi:10.5923/j.ajps.20160601.03

Characterization of a Biodegradable Polyurethane Elastomer Derived from Castor Oil
Fereshteh Abdolhosseini1, Mohammad Kazem Besharati Givi2
1Department of Textile Chemistry, University of Kar, Tehran, Iran
2Department of Material and Mechanical Engineering, University of Tehran, Tehran, Iran
Correspondence to: Fereshteh Abdolhosseini, Department of Textile Chemistry, University of Kar, Tehran, Iran.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Vegetable oils are considered the renewable raw materials with a good functionality, the most abundant and the cheapest, to produce the polyurethane (PU). The castor oil with high degree of unsaturation and low hydroxyl functionality, can act as a bio-based polyol to synthesize the PU. In this project, the structure of this oil is characterized and the percentage of ricinoleic acid was estimated by the reaction with a monoisocyanate and FTIR analysis. PU elastomer was prepared from castor oil (CO) with a petrochemical derived aliphatic diisocyanate (IPDI), in the presence of catalyst, and the condition of stoichiometry. The rheological properties were analyzed for knowledge of the evolution of the polymeric mixture viscosity. The elastomer obtained showed good thermal and mechanical properties. The PU based castor oil had low degree of water uptake, and relatively good degree of toluene swelling.
Keywords: Bio-based polyurethane, Fatty acid, Castor oil, Elastomer
Cite this paper: Fereshteh Abdolhosseini, Mohammad Kazem Besharati Givi, Characterization of a Biodegradable Polyurethane Elastomer Derived from Castor Oil, American Journal of Polymer Science, Vol. 6 No. 1, 2016, pp. 18-27. doi: 10.5923/j.ajps.20160601.03.
Article Outline
1. Introduction
- Polyurethane is known as one of the materials having great importance in the industry with excellent properties. Polyurethanes have broad applications in many different fields such as foams (flexible, semi-rigid and rigid), elastomers, adhesives, coatings and fibers [1-5]. The polyurethanes are obtained by the polyaddition reaction of polyols and polyisocyanates [6, 7]. The obtained polyurethane properties depend on the molecular weight, degree of crosslinking, the ratio of NCO/OH, effective intermolecular forces and the stiffness of different chain segments [8, 9]. The polyurethane chain is composed of a soft segment derived from polyol and a hard segment derived from diisocyanate and a chain extender [10-12] (Figure 1).
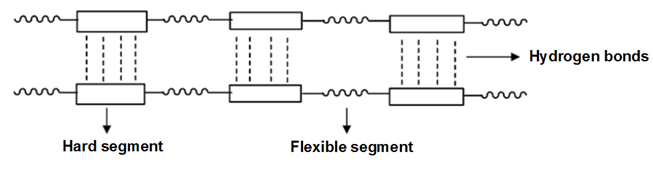 | Figure 1. Linear segmented polyurethane chain structure |
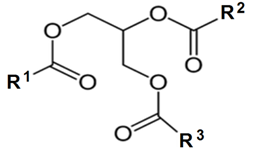 | Figure 2. Structure of a triglyceride with fatty acid (R1, R2 and R3) |
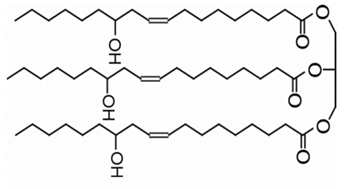 | Figure 3. Chemical formula of castor oil |
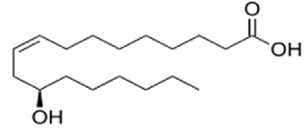 | Figure 4. Structure of ricinoleic fatty acid |
2. Experimental
2.1. Materials
- The Castor oil (purity 80% and Mw= 932 gr.mol-1) was purchased from the Merck Sigma-Aldrich in Germany. In addition, the Cyclohexyl isocyanate (Mw=125.17 gr.mol1), the Isophoron diisocyanate (3-isocyanatomethyl-3, 5, 5-trimethylcyclohexyle) _ with the commercially name of IPDI (Mw=222.29 gr.mol-1), the Dibutyl tin dilaurate known as the catalyst _with the commercially name of DBDL (Mw=631.55 gr.mol-1) _, the Potassium hydroxide (≥85% KOH) and the Toluene (≥99.8%, anhydrous) were provided by Sigma Aldrich company. The high reactivity of the hydroxyl group leads to the absorption of the water molecules from the surrounding environment. During the polymerization, a portion of the NCO functional groups reacts with the molecules of water. To avoid these secondary reactions, it is necessary to eliminate moisture in castor oil, before use [8, 17, 29, 30]. Castor oil was dried in a vacuum oven at 110°C for 12h.
2.2. The Polyurethane Synthesis
2.2.1. The Synthesis with Monoisocyanate
- Considering that the castor oil is completely pure (100%), 7.3 gr of castor oil was mixed with 3.1 gr of IPDI and also, 0.2 wt. % of total weight of the mixture, the catalyst was added into a three-necked flask, at the room temperature for 8 hours, under a dry nitrogen.It should be reminded that the monoisocyanate used in this mixture got exceeded about 10% more than of the weight counted in the stoichiometric, in order to determine the NCO rate used by the excessive amount of isocyanate found in the spectrum obtained by infrared [31, 32].
2.2.2. The Synthesis with Diisocyanate
- In this research, the NCO/OH ratio was 1, indicates that [NCO] = [OH] during polymerization [31]. 2.82 gr of Isophoron diiocyanate was mixed with 7.17 gr of castor oil (with purity of 80%) in stoichiometric: n (OH) = n (NCO) and with the catalyst at 0.2 wt. % of mixture, with regard to the total weight of castor oil and the IPDI in thermostat oil tub at 40°C, in a dry nitrogen and agitator of 200 rpm.min-1, for 30 minutes. The polymer mixture resulted a viscose solution and it was poured out onto a teflon plate of 2mm thickness. The plate was put in a vacuum oven at 100°C, for 24 hours to get dried.
2.3. Measurements
2.3.1. Characteristics of castor oil
- The hydroxyl value of castor oil was determined according to ASTM D1957-86.Hydroxyl functionality, which is the number of hydroxyl group per molecule, was experimentally calculated by the following formula [3]:
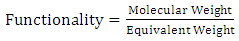 | (1) |
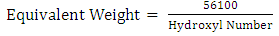 | (2) |
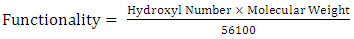 | (3) |
|
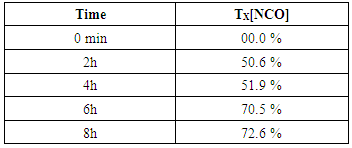
2.3.2. Consumption rate of NCO Group Oil
- Evolution of transformation rate of isocyanate group over time will be obtained by using the following formula [10]:
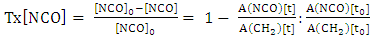 | (4) |
2.3.3. Purity of Castor Oil
- Once the isocyanate consumes rate is determined from the amount of excess isocyanate observed in the spectrum, it deduces the amount of alcohol functions in the oil and therefore its purity.The weight and the percentage of ricinoleic acid in the castor oil is calculated by the following experimental formulas obtained during the experiments:
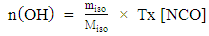 | (5) |
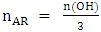 | (6) |
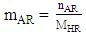 | (7) |
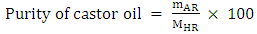 | (8) |
2.4. Analysis
2.4.1. The Fourier Transform Infrared Spectroscopy
- This analysis is performed using a Thermo Electron Corporation spectrometer (IR 200 Series). The range of this analysis extends from 4000 to 500cm-1. The spectra were obtained using the OMNIC 7.3 software which also allows measuring the peak area. A background is done before each absorbance spectrum for reduce unwanted peaks in the spectrum by placing the pellets KBr. These pellets are prepared by hydraulic press of fine powder of potassium bromide, between two cylinders.The FTIR analysis of the mixture is carried out at different time points in order to follow the progress of the chemical reaction. The spectrum is introduced a total absorbance value.
2.4.2. Rheology
- Rheology is the study of polymer flow. The viscosity of polyurethane mixture, obtained by castor oil and IPDI, was determined by the remoter AR 2000 ex TA Instruments; a percentage of strain of 1%, a 10Hz scan rate and a 4 mm gap were chosen. The evaluation of the polyurethane viscosity was accomplished, with regard to the temperature at 25°C and 50°C, and also by adding a concentration of 0.2 wt. % of catalyst.
2.4.3. Water uptake
- The water absorption of the polyurethane was measured by immersing the polyurethane film (10mm×10mm×2mm) in 50mL of water, at 30°C in a humidified incubator, during 14 days. After the time interval, the polyurethane film was removed from the water, and also the excess water on the surface was removed; further, the polyurethane film was weighed with an analytical balance. The percentage of water absorption was calculated according to the following formula [6, 8, 22]:
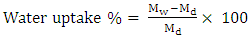 | (9) |
 and
and 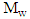 are respectively the weights of dry and wet sample.
are respectively the weights of dry and wet sample.2.4.4. Swelling
- The equilibrium swelling was carried out by immersing the polyurethane film in Toluene at 30°C, during 72h. The measure of the weights of swollen polyurethane film was carried out in every 4 hours during the first 24 hours and continued in every 12h, until finding the constant weight. The equilibrium degree of swelling (Q) was calculated by following formula [15]:
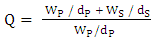 | (10) |
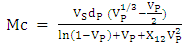 | (11) |
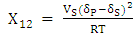 | (12) |
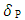 and
and 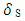 are the solubility parameters of the polymer and solvent. Toluene solubility parameter is 18.2 (J.cm-3)1/2 [6] and the polyurethane solubility parameter was determined by the cohesive energy of the system in swelling test according to the Eq. 10. Eventually, the crosslinking density was calculated according to the following formula [26]:
are the solubility parameters of the polymer and solvent. Toluene solubility parameter is 18.2 (J.cm-3)1/2 [6] and the polyurethane solubility parameter was determined by the cohesive energy of the system in swelling test according to the Eq. 10. Eventually, the crosslinking density was calculated according to the following formula [26]: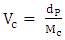 | (13) |
2.4.5. Mechanical Properties
- The mechanical properties were determined according to ASTM D638. The mechanical testing was performed by using an MTS tensile tester (USA). The measurements were performed at room temperature with a load cell (load one) of 500N and crosshead speed (crosshead speed) of 50 mm/min. All Tensile strength (σ), elongation at break (ε), and the Young's modulus (E) were measured with the polyurethane film with dimension 70mm × 25mm × 2mm (length × width × thickness). The data presented are an average of 4 different measures and they were recorded by the QT TestWorks software.
2.4.6. Thermal Analysis (DSC)
- Differential scanning calorimetry test was performed on a TA Instruments 2920 machine. The analysis was made with a sample weight of 10 mg, placed in aluminum capsules. The Scanning is carried out in temperature from -20°C to 220°C under nitrogen. The heating and cooling speed was set at 10°C/min.
3. Results and Discussion
3.1. FTIR
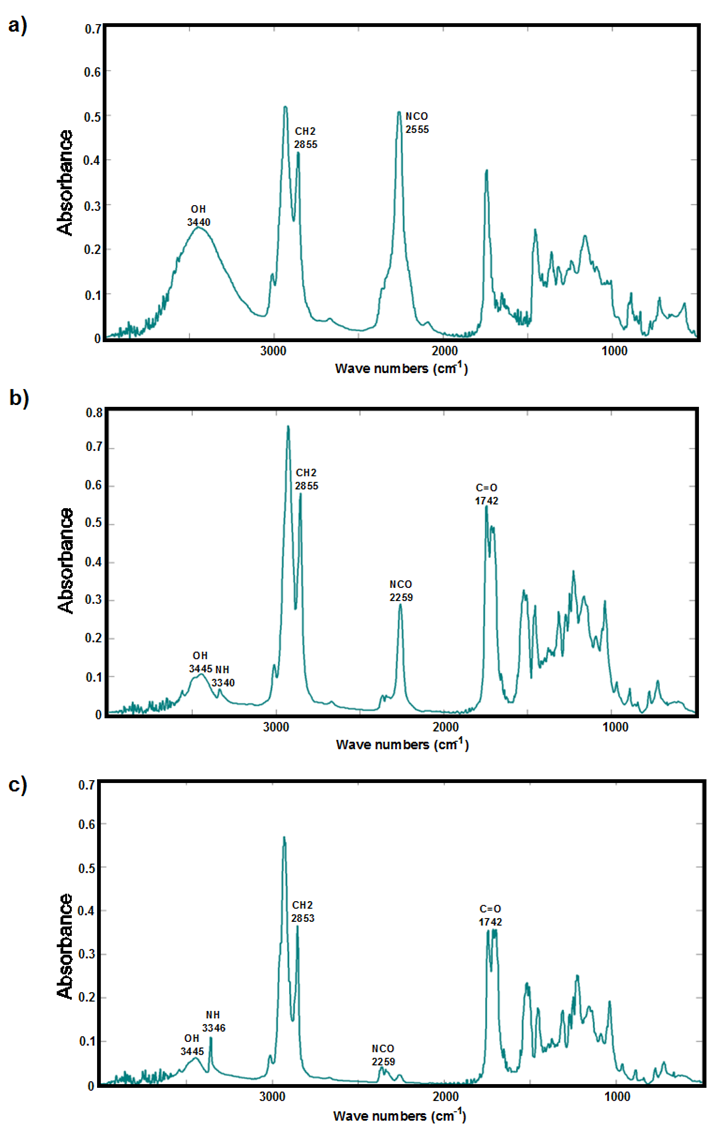
- The infrared spectrum of the Bio-based polyurethane, based on Castor oil and the Cyclohexyl isocyanate, in the initial, middle and final time, were shown in Figure 5. Broadband between 3200 cm-1 and 3600 cm-1, corresponded to the OH functions existing in castor oil. The stretching vibration band of the NCO function of the isocyanate was evident between 2250 cm-1 and 2400 cm-1. The bands on the stretching vibration of the CH2 group and the carbonyl group (C = O) of the urethane bond are observed at 2855 cm-1 and 1742 cm-1, respectively. Reducing progressively the band of the NCO group noted the progress of the reaction between the hydroxyl function and isocyanate, during polymerization. In parallel this event, a new band appeared at 3340 cm-1 which corresponds to the NH stretching vibration. The establishment of hydrogen bonds between the NH function, the C = O group, and an oxygen castor oil, leading to the formation of a urethane bond (-NH-COO-) in the bio-based polyurethane structure. The small NCO band observed in the spectrum at the end of polymerization, corresponds to the excess isocyanate which remains without reacting with the OH of the castor oil functions.
|
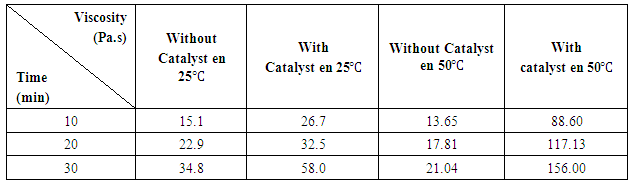
3.2. Viscosity
- The study of rheological properties, initially focuses on the viscosity evaluation of the polyurethane system according to the temperature and depending on the catalyst, as it allows to know the progress of the reaction. Figure 6 shows that the increase of the temperature of a polyurethane system slowly decreases the viscosity of the polymer in a short time. It should be noted that the increase of PU mixture temperature in a long period of time leads to increase speed of polymerization. So, the concentration of 0.2% catalyst had a great importance on the reactivity between castor oil and IPDI, therefore, a higher viscosity is evident. The reaction started quickly and the growth of the chains leads to an increase of the viscosity.
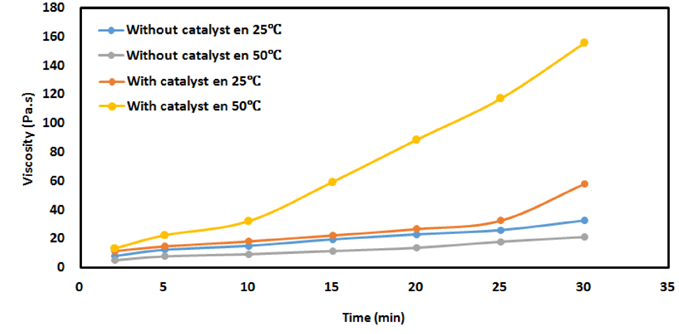 | Figure 6. Evolution of the viscosity of the mixture castor oil/IPDI according to time and catalyst |
|
3.3. Water Uptake
- The water absorption is one of the polyurethane physical properties, which is of great importance to the durability of the polymer. Because the polyurethane degradation rate increases with the absorption of the more water. Bio-based Polyurethane, based on castor oil and IPDI, indicates a per-centage of water absorption equal to 2.36% which corresponds to a hydrophobic nature of this PU. The hydrocarbon chains of racinoleic acid, in the castor oil structure, are the big aliphatic chains which make the lower penetration of water molecules in the PU network. This result shows that the PU based on the castor oil has a good resistance to water degradation.
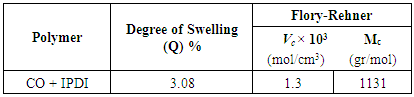
3.4. Swelling and Cross-Linking Density
- The swelling test is a method used to measure the crosslinking density, which is calculated according to the Flory-Rehner theory based on affine network. The crosslinking density is defined by the number of crosslinking per unit volume [15]. The results obtained for the degree of swelling (Q), the crosslinking density (VC), and the average molecular weight between two cross-links (MC) are shown in Table 4.
|
|
3.5. Mechanical Properties
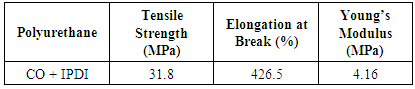
- The stress-strain curve obtained by the tensile test for bio-based polyurethane which is set forth in Figure 7. Table 5 shows the results of this curve for the polymer mechanical properties. The polymer structure, the ratio of NCO/OH and the degree of crosslinking [5, 15] are the most effective elements on the mechanical properties. Castor oil naturally contain 80% ricinoleic acid and the hydroxyl groups distributed heterogeneous on its chain triglyceride [3], which leads to the network affinity in the final polyurethane structure, which corresponds to good mechanical properties. In the NCO/OH =1 ratio, the use of castor oil with a high degree of unsaturation, with an aliphatic disocyanate (IPDI), gives relatively good tensile strength. High elongation at break of polymer defined by the long chain aliphatic fatty acid in castor oil [4] and the average molecular weight between two cross-links (MC) which derive from the flexibility of the obtained polyurethane chains. The Low Young's modulus of polyurethane corresponds to the low hydroxyl functionality of castor oil. The young's modulus also affects the glass transition temperature (Tg) of PU, and low modulus decreases the Tg. higher elongation at break and lower Young's modulus was observed, and according to the level of obtained tensile strength, it corresponds to a semi-rigid elastomer.
3.6. DSC
- The differential scanning calorimetry is one of the techniques for measuring the glass transition temperature (Tg) of polyurethane elastomer. The Figure 8 presents the thermal behavior of polyurethane elastomer synthesized.The cross linking density [2, 29, 46], crystallinity [26], hydroxyl number and polyol functionality are the most important parameters on the glass transition temperature (Tg). By reducing hydroxyl number and functionality, the crosslinking density and therefore, the crystalline phase decreases which causes a low Tg [35]. Castor oil has an amorphous structure with a hydroxyl number of 163 and a low hydroxyl functions leads to low crosslinking density.
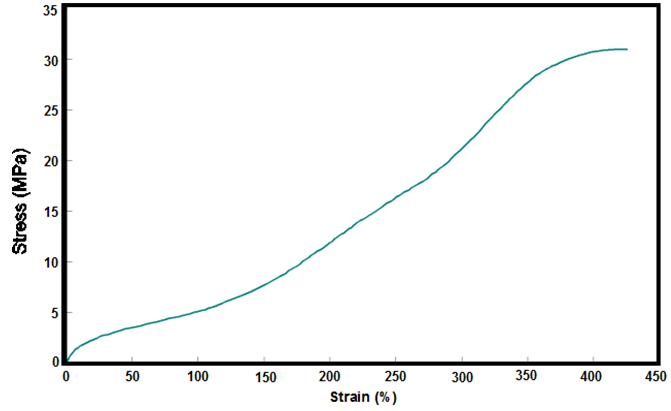 | Figure 7. Stress-strain curve for bio-based polyurethane elastomer made from castor oil |
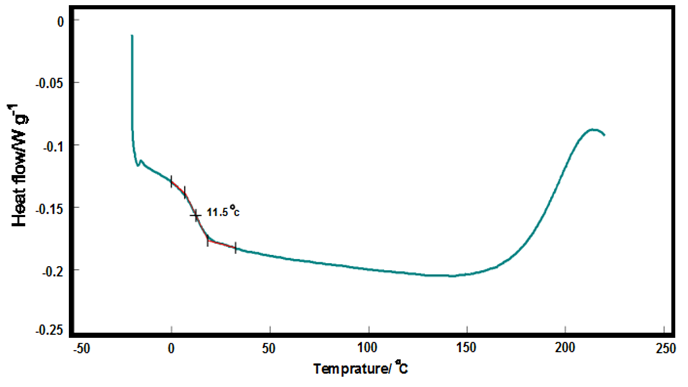 | Figure 8. DSC curve of bio-based polyurethanes made from castor oil |
4. Conclusions
- Castor oil with hydroxyl functionality of 2.67 and hydroxyl number of 163 has been used as a bio-based polyol to synthesize a new polyurethane elastomer. The presence and the structure of ricinoleic acid in this oil is of great importance on the final polymer properties. The FT-IR spectrum determines the ricinoleic acid poucentage (≅80%). This oil has a high degree of unsaturation which leads to good mechanical properties. The castor oil based PU elastomer shows a relatively good tensile strength (31.8 MPa), while it also has a high elongation at break (426.5%) drive from the large fatty acid chain. The presence of a low cross-linking density confirmed in the polyurethane elastomer, according to the Flory-Rehner; and it showed a hydrophobic nature according to the water uptake test. The reason for this discrepancy in the physical properties can be explained by homogeneous distribution of the hydroxyl groups on the chains in castor oil, thanks to a uniform structure of triglycerides, which leads to the affinity network. By creating this affinity network and having a higher degree of unsaturation of castor oil, a relatively high Tg (preferably less than room temperature) is a evident for bio-based PU system; regarding the chain mobility in the structure final elastomer. These properties obtained can be attributed to training a semi-rigid elastomer.
Abbreviations

 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML