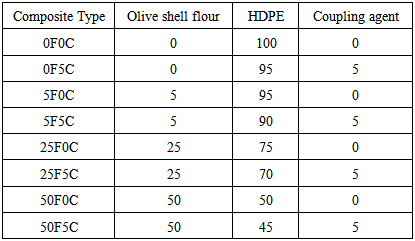
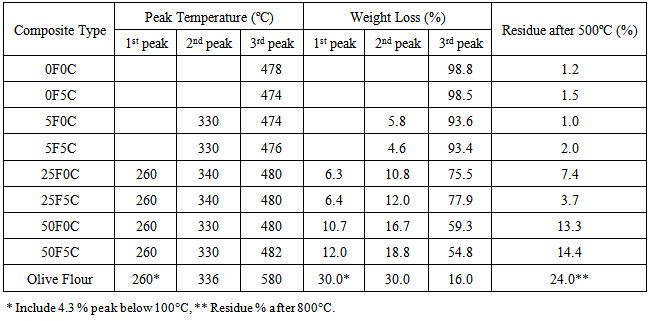
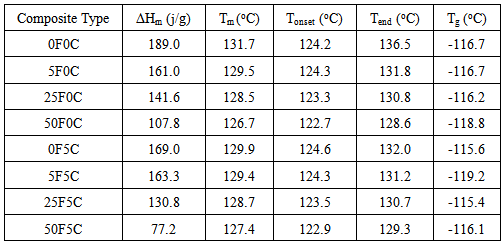
| [1] | Clemons, C.M. Wood flour, Functional fillers for plastics, M. Xanthos 9(ed.), 2nd Ed., Wiley-VCH Verlag GmBH & Co., Weinheim, Germany; 2010. pp. 260-290. |
| [2] | Berglund, L. and Rowell, R.M. Wood composites, handbook of wood chemistry and wood composites. CRC Press, Boca Raton, Florida; 2005. |
| [3] | Carlborn, K., and Matuana, L. M., 2006, Functionalization of wood particles through a reactive extrusion process. J. Appl. Polym. Sci., 101(5), 3131-3142. |
| [4] | Ashori, A., Sheshmani, S., and Farhani, F., 2013, Preparation and characterization of bagasse/HDPE composites using multi-walled carbon nanotubes. Carb. Polym., 92(1), 865-871. |
| [5] | Stark, N. M., and Mueller, S. A., 2008, Improving the color of stability of wood-plastic composites through fiber pre-treatment. Wood Fiber Sci., 40(2), 271-278. |
| [6] | Ayrilmis, N., Dundar, D., Kaymakci, A., Ozdemir, F., Kwon J. H., 2014, Mechanical and thermal properties of wood-plastic composites reinforced with hexagonal boron nitride. Polm. Compos, 194–200. |
| [7] | Tisserat, B., Reifschneider, L., Joshee, N., and Finkenstdt, V. L., 2013, Properties of high density polyethylene- paulownia wood flour composites via injection molding. Bioresources, 8 (3), 4440-4458. |
| [8] | Mohammadi-Rovshandeh, J., Pouresmaeel-Selakjani, P., Davachi, S.M., Kaffashi, B., Hassani, A., Bahmeyi, A., 2014, Effect of lignin removal on mechanical, thermal, and morphological properties of polylactide/starch/rice husk blend used in food packaging. J. Appl. Polym. Sci., 131, 41095. |
| [9] | Liu, H., Wua, Q., Zhang, Q., 2009, Preparation and properties of banana fiber-reinforced composites based on high density polyethylene (HDPE)/Nylon-6 blends. Bioresource Technology, 100, 6088–6097. |
| [10] | Myers, G. E., Chahyadi, I. S., Gonzalez, C., Coberly, C. A., and Ermer, D. S., 1991, Wood flour and polypropylene or high-density polyethylene composites: Influence of maleated polypropylene concentration and extrusion temperature on properties. Intern. J. Polym. Mater. 15(3-4), 171-186. |
| [11] | Wollerdorfer, M. Bader, H., 1998, Influence of natural fibers on the mechanical properties of biodegradable polymers. Ind Crop Prod., 8, 105–112. |
| [12] | Sewda, K., Maiti, S. N., 2009, Mechanical properties of teak wood flour-reinforced HDPE composites. J. App. Polym. Sci., 112, 1826–1834. |
| [13] | El Nemr, M. H., El Shazly, Y. M. S., Mubarak, A. A., Zaki, M. Z., 2015, Composites from rice straw and high density polyethylene: thermal and mechanical properties. Int. j. eng. sci., 4(4), 57-64. |
| [14] | Kalia S, Kaith BS, Kaurs I, Eds., Cellulose fibers: bio- and nano- polymer composites. New York: Springer; 2011. |
| [15] | Jawaid M, Abdul Khalil HPS., 2011, Cellulosic/synthetic fiber reinforced polymer hybrid composites: A review. Carbohydrate Polym. 86(1):1–18. |
| [16] | Kim, H. S., Yang, H. S., Kim H. J., and Park, H. J., 2011, Thermogravimetric analysis of rice husk flour filled thermoplastic polymer composites. J. Therm. Anal. Cal., 76, 395–404. |
| [17] | Naghmouchi, I., Mutje, P., Boufi, S., 2014, Polyvinyl chloride composites filled with olive stone flour: mechanical, thermal, and water absorption properties. J. Appl. Polym. Sci., 131, 41083. |
| [18] | Kaboorani, A., and Faezipour, M., 2009, Effects of wood preheat treatment on thermal stability of HDPE Composites. Journal of Reinforced and Plastic Composites, 28: 24. |
| [19] | Farhadinejad, Z., Ehsani, M., Khosravian, B., Ebrahimi, G., 2012, Study of thermal properties of wood plastic composite reinforced with cellulose micro fibril and nano inorganic fiber filler. Eur. J. Wood Prod., 70: 823–828. |
| [20] | Suñol J. J., and Saurina, J., 2002, Thermal analysis of aged HDPE based composites. J. Therm. Anal. Cal., 70, 57-62. |
| [21] | Mengeloglu, F., and Kabakci, A., 2008, Determination of thermal properties and morphology of eucalyptus wood residue filled high density polyethylene composites. Int. J. Mol. Sci., 9, 107-119. |
| [22] | Monteiro, S. N., Calado, V., Rodriguez, R.J.S., Margem, F.M., 2012, Thermogravimetric stability of polymer composites reinforced with less common lignocellulosic fibers – an overview. J. Mater. Res. Tecnol., 1(2):117-126. |
| [23] | Ndiaye, D., and Tidjani, A., 2012, Effects of coupling agents on thermal behavior and mechanical properties of wood flour/polypropylene composites. Journal of Composite Materials, 46(24) 3067–3075. |
| [24] | Kaboorani, A., 2010, Effects of formulation design on thermal properties of wood/thermoplastic composites. Journal of Composite Materials, 44, 18. |
| [25] | Mengeloglu, F., and Karakus, K., 2008, Thermal degradation, mechanical properties and morphology of wheat straw flour filled recycled thermoplastic composites. Sensors, 8, 500-519. |
| [26] | Moriana, R., Vilaplana, F., Karlsson, S., Ribes-Greus, A., 2011, Improved thermo-mechanical properties by the addition of natural fibers in starch-based sustainable biocomposites. Composites Part A., 42:30–40. |
| [27] | Julkapli NM, Akil HM., 2010, Thermal properties of kenaf-filled chitosan biocomposites. Polym-Plast Technol Eng., 49(2):147–53. |
| [28] | Akil, H.M., Omar, M.F., Mazuki, A.A.M., Safiee, S., ZAM, I., Bakar A.A., 2011, Kenaf fiber reinforced composites: A review. Mater Design, 32(8-9):4107–21. |
| [29] | Mirabella, F. M. and Bafna, A., 2002, Determination of crystallinity of polyethylene/&alpha,-olefin copolymers by thermal analysis relationship of the heat of fusion of 100% polyethylene crystal and the density. J. Polym. Sci. Polym. Phys., 40, 1637-1643. |
| [30] | Mohanty, S., Verma, SK., Nayak, SK., 2006, Dyamic mechanical and thermal properties of MAPE treated jute/HDPE composites. Compos Sci. Technol., 66: 538–47. |
| [31] | Doan, T.T.L., Brodowsky, H., Mäder, E., 2007, Jute fibre/polypropylene composites. II. Thermal, hydrothermal and dynamic mechanical behavior. Compos Sci. Technol., 67: 2707–14. |
| [32] | Sebio-Punal, T., Naya, S., Lopez-Beceiro, J., Tarrıo-Saavedra, J., Artiaga, R., 2012, Thermogravimetric analysis of wood, holocellulose, and lignin from five wood species. J. Therm. Anal. Cal., 109(3), 1163-7. |
| [33] | Iulianelli, G. C. V.; Maciel, P. M. C.; Tavares, M. I. B. 2011, Preparation and characterization of PVC/natural filler composite. Macromol. Symp., 299-300 (1), 227-233. |
| [34] | McNeill, I., Memetea, L., Cole, W., 1995, A study of the product of PVC thermal degradation. Polym. Degrad. Stab., 49(1), 181. |
| [35] | Purohit, V., Orzel RA., 1988, Polypropylene: a literature review of the thermal decomposition products and toxicity. Int J Toxicol., 7: 221–242. |
| [36] | Enayati, AA., Hosseinaei, O., Wang, S., Mirshokraie, SA., Tajvidi, M., 2009, Thermal properties of wood–plastic composites prepared from hemicellulose-extracted wood flour. Iranian J Polym Sci Tech., 3:171–181. |
| [37] | Clemons, CM., 2002, Wood–plastic composites in the United States: the interfacing of two industries. Forest Prod J., 52:10–18. |
| [38] | Li, B., He, J., 2004, Investigation of mechanical property, flame retardancy and thermal degradation of LLDPE-wood-fibre composites. Polym. Degrad. Stabil., 83, 241-246; doi 10.1016/S0141- 3910(03)00268-4. |
| [39] | Amen-Chen, C., 2001, Production of monomeric phenols by thermochemical conversion of biomass: a review. Bioresour. Technol., 79, 277-299; doi: 10.1016/S0960 8524(00)00180-2; PubMed 11499582. |
| [40] | Wikberg, H., and Mauna SL., 2004, Characterisation of thermally modified hard and softwoods by 13C CPMAS NMR. Carbohyd Polym., 58: 461–466. |
| [41] | Khalaf, M.N., 2010, The effect of alkali lignin on heat of fusion, crystallinity and melting points of low density polyethylene (LDPE), medium density polyethylene (MDPE), and high density polyethylene (HDPE). J. Thi-Qar Sci., 2(2), 89-95. |
| [42] | Gao, M., Zhu, K., and Sun, Y.J., 2004, Thermal degradation of wood treated with amino resins and amino resins modified with phosphate in nitrogen. J Fire Sci., 22: 505-515. |

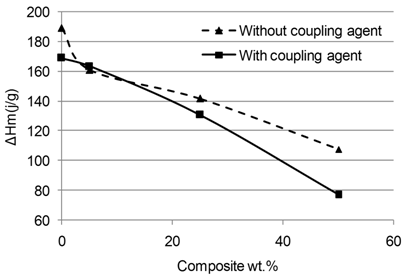
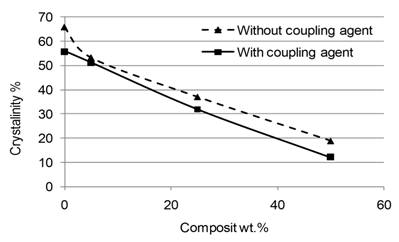
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML