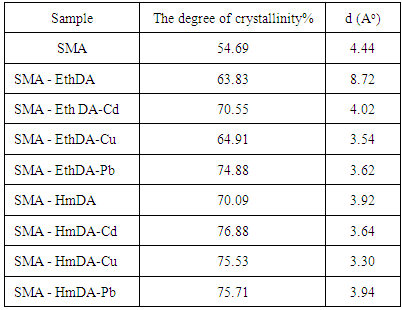
| [1] | Nwuche, C.O., and Ugoji, E.O., 2008, Effects of heavy metal pollution on the soil microbial activity, Int. J. Environ. Sci. Technol. 5(3), 409–414. |
| [2] | Sternberg, S.P.K., and Dorn, R.W., 2002, Cadmium removal using Cladophora in batch, semi-batch and flow reactors, Bioresource Technology 81(3), 249–255. |
| [3] | Liang, X., Su, Y., Yang, Y., and Qin, W., 2012, Separation and recovery of lead from a low concentration solution of lead (II) and zinc (II) using the hydrolysis production of poly styrene-co-maleic anhydride, Journal of hazardous materials 203, 183–187. |
| [4] | Bowen, Humphry John Moule, Environmental chemistry of the elements, Academic Press, 1979. |
| [5] | Yu, B., Zhang, Y., Shukla, A., Shukla, S.S., and Dorris, K.L., 2001, The removal of heavy metals from aqueous solutions by sawdust adsorption—removal of lead and comparison of its adsorption with copper, Journal of hazardous materials 84(1), 83–94. |
| [6] | Leyva-Ramos, R., Rangel-Mendez JR, Mendoza-Barron, J., Fuentes-Rubio, L., and Guerrero-Coronado, R.M., 1997, Adsorption of cadmium (II) from aqueous solution onto activated carbon, Water Science and Technology 35(7), 205–211. |
| [7] | Emongor, V., 2007, Biosorption of lead from aqueous solutions of varied pH by kale plants (Brasicca oleraceae varacephala), Journal of Agricultural, Food and Environmental Sciences 1(2), 90–91. |
| [8] | Madadrang, C.J., Kim, H.Y., Gao, G., Wang, N., Zhu, J., Feng, H., Gorring, M., Kasner, M.L., Hou, S.F, 2012, Adsorption behavior of EDTA-graphene oxide for Pb (II) removal, ACS Appl. Mater. Interfaces 4, 1186–1193. |
| [9] | Liu, D.G., Li, Z.H., Li, W., Zhong, Z.R., Xu, J.Q. J.J. Ren, Z.S. Ma, 2013, Adsorption behavior of heavy metal ions from aqueous solution by soy protein hollow microspheres, Ind. Eng. Chem. Res. 52, 11036–11044. |
| [10] | Zhao, G., Wu, X., Tan, X., and Wang, X., 2011, Sorption of heavy metal ions from aqueous solutions: a review, Open Colloid Science Journal 4, 19–31. |
| [11] | Chen, J. H., Hsu, K.C., Chang, Y. M., 2013, Surface modification of hydrophobic resinwith tricaprylmethylammonium chloride for the removal of trace hexavalent chromium, Ind. Eng. Chem. Res. 52 (2013) 11685–11694 |
| [12] | Othman, M.K., Al-Qadri, F.A., and Al-Yusufy, F.A., 2011, Synthesis, physical studies and uptake behavior of: Copper (II) and lead (II) by Schiff base chelating resins, Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 78(5), 1342–1348. |
| [13] | Wang, Y.P., Shen, Y.Q., Pei, X.W., Zhang, S.C., Liu, H.G., Ren, J.M., In situ synthesis of poly(styrene-co-maleic anhydride)/SiO2 hybrid composites via ‘grafting onto’strategy based on nitroxide-mediated radical polymerization, React. Funct. Polym. 68 (2008) 1225–1230. |
| [14] | Krüger, S., Krahl, F., Arndt, K.F., 2010, Random cross-linked poly(styrene-co-maleicanhydride): characterization of cross-linking intermediates by size exclusion chromatography, Eur. Polym. J. 46, 1040–1048. |
| [15] | Qi, X.-H., Jia, X.-Q., Ying, Y., Niu, L.-E., and Hou, L.-P., 2010, Recovery of nickel from mixed solution containing light metals by PSt/MA resin, Transactions of Nonferrous Metals Society of China 20, s102. |
| [16] | McClain, A.R., and Hsieh, Y.-L., 2004, Synthesis and metal complexation of dihydroxyphosphino-functionalized crosslinked styrene/maleic anhydride copolymers, J. Polym. Sci. A Polym. Chem. 42(1), 92–101. |
| [17] | Lin, J.-J., and Hsu, Y.-C., 2009, Temperature and pH-responsive properties of poly (styrene-co-maleic anhydride)-grafting poly (oxypropylene)-amines, Journal of colloid and interface science 336(1), 82–89. |
| [18] | Gonte, R.R., and Balasubramanian, K., 2012, Chemically modified polymer beads for sorption of gold from waste gold solution, Journal of hazardous materials 217, 447–451. |
| [19] | Zhu, Z., Sun, F., Yang, L., Gu, K., Li, W., 2013, Poly (styrene-co-maleic anhydride) microspheres prepared in ethanol/ water using a photochemical method and their application in Ni2+adsorption, Chemical Engineering Journal 223, 395–401. |
| [20] | Wei, K., Wu, J., Chen, Y., Hsu, Y., and Lin, J., 2007, Easy preparation of crosslinked polymer films from polyoxyalkylene diamine and poly (styrene–maleic anhydride) for electrostatic dissipation, Journal of applied polymer science 103(2), 716–723. |
| [21] | Ma, X., Li, L., Yang, L., Su, C., Wang, K., Yuan, S., and Zhou, J., 2012, Adsorption of heavy metal ions using hierarchical CaCO 3–maltose meso/macroporous hybrid materials: Adsorption isotherms and kinetic studies, Journal of hazardous materials 209, 467–477. |
| [22] | Bruch, M., Mäder, D., Bauers, F., Loontjens, T., and Mülhaupt, R., 2000, Melt modification of poly (styrene‐co‐maleic anhydride) with alcohols in the presence of 1, 3‐oxazolines, Journal of Polymer Science Part A: Polymer Chemistry 38(8), 1222–1231. |
| [23] | Haider, S., and Park, S.-Y., 2009, Preparation of the electrospun chitosan nanofibers and their applications to the adsorption of Cu (II) and Pb (II) ions from an aqueous solution, Journal of Membrane Science 328(1), 90–96. |
| [24] | Ngah, W.W., Endud, C.S., and Mayanar, R., 2002, Removal of copper (II) ions from aqueous solution onto chitosan and cross-linked chitosan beads, Reactive and Functional Polymers 50(2), 181–190. |
| [25] | Guibal, E., Ruiz, M., Vincent, T., Sastre, A., and Navarro-Mendoza, R., 2001, Platinum and palladium sorption on chitosan derivatives, Separation Science and Technology 36(5-6), 1017–1040. |
| [26] | Justi, K.C., Fávere, V.T., Laranjeira, M.C.M., Neves, A., and Peralta, R.A., 2005, Kinetics and equilibrium adsorption of Cu (II), Cd (II), and Ni (II) ions by chitosan functionalized with 2 [-bis-(pyridylmethyl)aminomethyl]-4-methyl-6-formylphenol, Journal of colloid and interface science 291(2), 369–374. |
| [27] | Wang, L., Xing, R., Liu, S., Yu, H., Qin, Y., Li, K., Feng, J., Li, R., and Li, P., 2010, Recovery of silver (I) using a thiourea-modified chitosan resin, Journal of hazardous materials 180(1), 577–582. |
| [28] | Ho, Y.-S., 2006, Second-order kinetic model for the sorption of cadmium onto tree fern: a comparison of linear and non-linear methods, Water research 40(1), 119–125. |
| [29] | Ho, Y.-S., and McKay, G., 2000, The kinetics of sorption of divalent metal ions onto sphagnum moss peat, Water research 34(3), 735–742. |
| [30] | Weber, W.J., and Morris, J.C., 1963, Kinetics of adsorption on carbon from solution, Journal of the Sanitary Engineering Division 89(2), 31–60. |
| [31] | Wang, P., Du, M., Zhu, H., Bao, S., Yang, T., and Zou, M., 2015, Structure regulation of silica nanotubes and their adsorption behaviors for heavy metal ions: pH effect, kinetics, isotherms and mechanism, Journal of hazardous materials 286, 533–544. |
| [32] | Donia, A.M., Atia, A.A., and Elwakeel, K.Z., 2008, Selective separation of mercury (II) using magnetic chitosan resin modified with Schiff's base derived from thiourea and glutaraldehyde, Journal of hazardous materials 151(2), 372–379. |
| [33] | Hua, M., Jiang, Y., Wu, B., Pan, B., Zhao, X., and Zhang, Q., 2013, Fabrication of a new hydrous Zr (IV) oxide-based nanocomposite for enhanced Pb (II) and Cd (II) removal from waters, ACS applied materials & interfaces 5(22), 12135–12142. |
| [34] | Weber, W.J., and Morris, J.C., 1962, Proceeding of the International Conference on Water Pollution Symposium, Pergamon Press, Oxford 2, 235–266. |
| [35] | Sadeghi, S., Rad, F.A., and Moghaddam, A.Z., 2014, A highly selective sorbent for removal of Cr (VI) from aqueous solutions based on Fe 3 O 4/poly (methyl methacrylate) grafted Tragacanth gum nanocomposite: Optimization by experimental design, Materials Science and Engineering: C 45, 136–145. |
| [36] | Mututuvari, T.M., and Tran, C.D., 2014, Synergistic adsorption of heavy metal ions and organic pollutants by supramolecular polysaccharide composite materials from cellulose, chitosan and crown ether, Journal of hazardous materials 264, 449–459. |
| [37] | Tan, I.A., Ahmad, A.L., and Hameed, B.H., 2009, Adsorption isotherms, kinetics, thermodynamics and desorption studies of 2, 4, 6-trichlorophenol on oil palm empty fruit bunch-based activated carbon, Journal of hazardous materials 164(2), 473–482. |
| [38] | Wang, J., Zhou, Y., Li, A., and Xu, L., 2010, Adsorption of humic acid by bi-functional resin JN-10 and the effect of alkali-earth metal ions on the adsorption, Journal of hazardous materials 176(1), 1018–1026. |
| [39] | Ramesh, A., Hasegawa, H., Sugimoto, W., Maki, T., and Ueda, K., 2008, Adsorption of gold (III), platinum (IV) and palladium (II) onto glycine modified crosslinked chitosan resin, Bioresource Technology 99(9), 3801–3809. |
| [40] | Wang, X., Zhang, Y., and Li, Q., 2011, Characterization and determination of the thermodynamic and kinetic properties of the adsorption of molybdenum (VI) onto microcrystalline anthracene modified with 8-hydroxyquinoline, Materials Science and Engineering: C 31(8), 1826–1831. |
| [41] | Hall, K.R., Eagleton, L.C., Acrivos, A., and Vermeulen, T., 1966, Pore-and solid-diffusion kinetics in fixed-bed adsorption under constant-pattern conditions, Industrial & Engineering Chemistry Fundamentals 5(2), 212–223. |
| [42] | Jin, X., Li, Y., Yu, C., Ma, Y., Yang, L., and Hu, H., 2011, Synthesis of novel inorganic–organic hybrid materials for simultaneous adsorption of metal ions and organic molecules in aqueous solution, Journal of hazardous materials 198, 247–256. |
| [43] | Chebotok, E.N., Novikov, V.Y., and Konovalova, I.N., 2007, Kinetics of base deacetylation of chitin and chitosan as influenced by their crystallinity, Russian Journal of Applied Chemistry 80(10), 1753–1758. |
| [44] | I. Boucher, K. Jamieson, A. Retnakaran, Advances in Chitin Science, Vol7.(Montreal: 9th ICCC), 2004. |

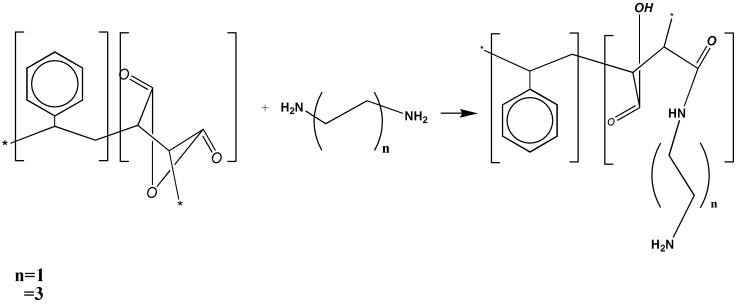
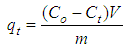
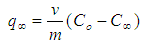
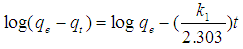
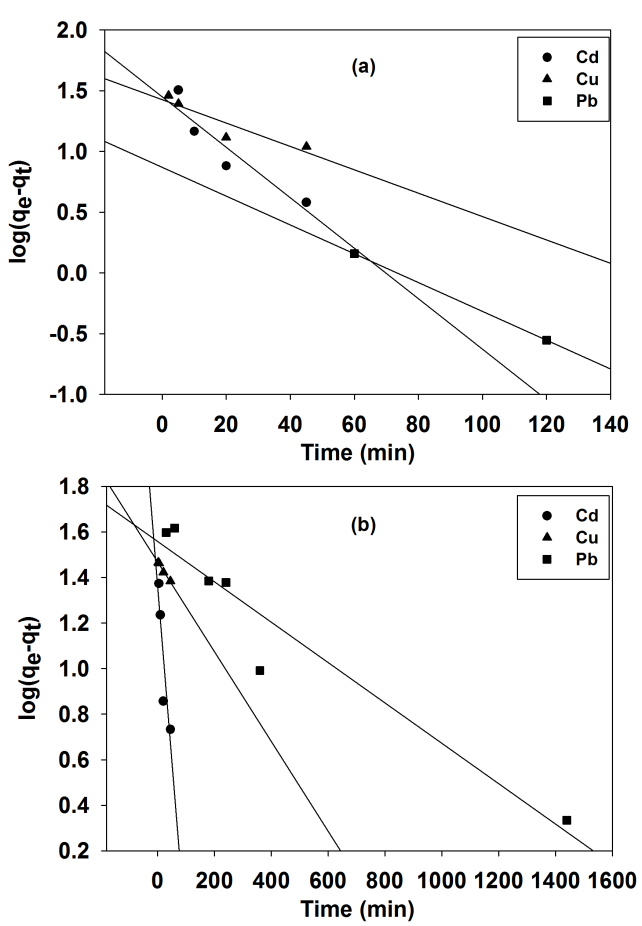
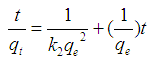
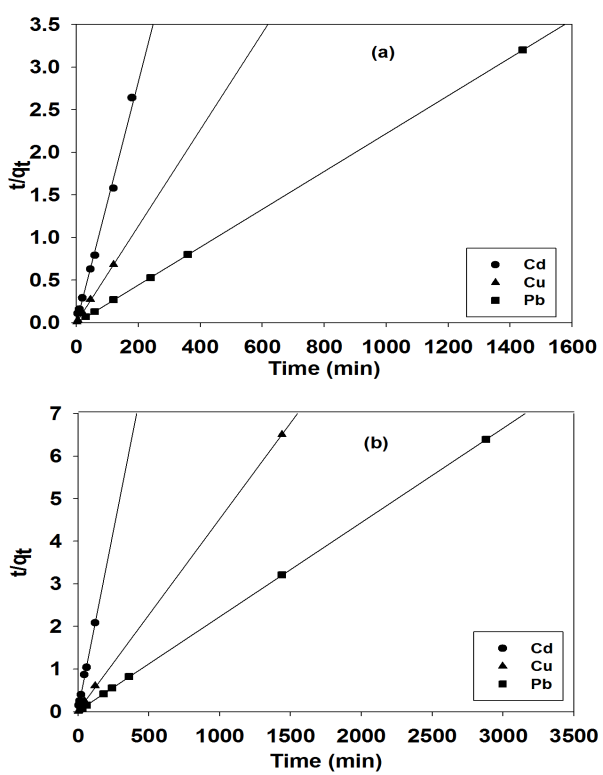

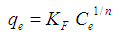
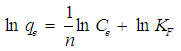
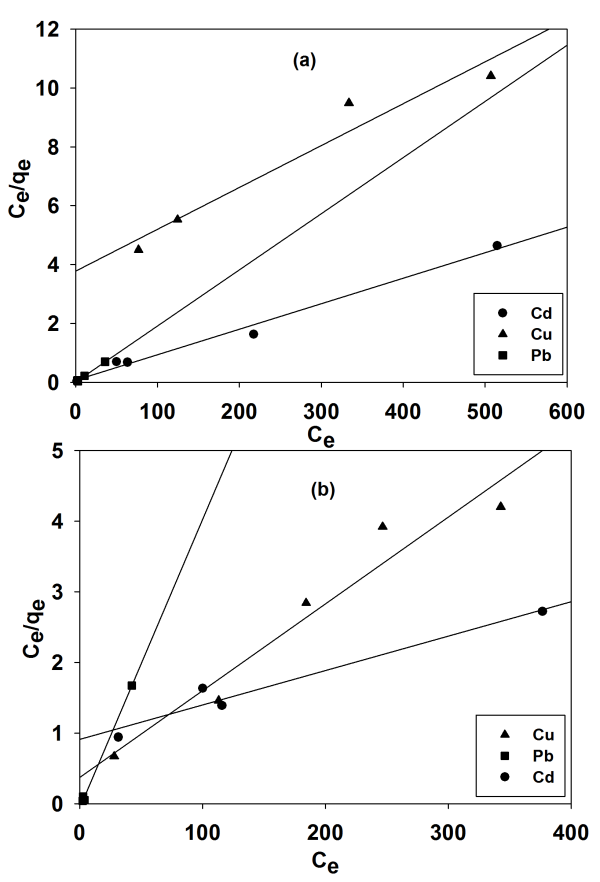
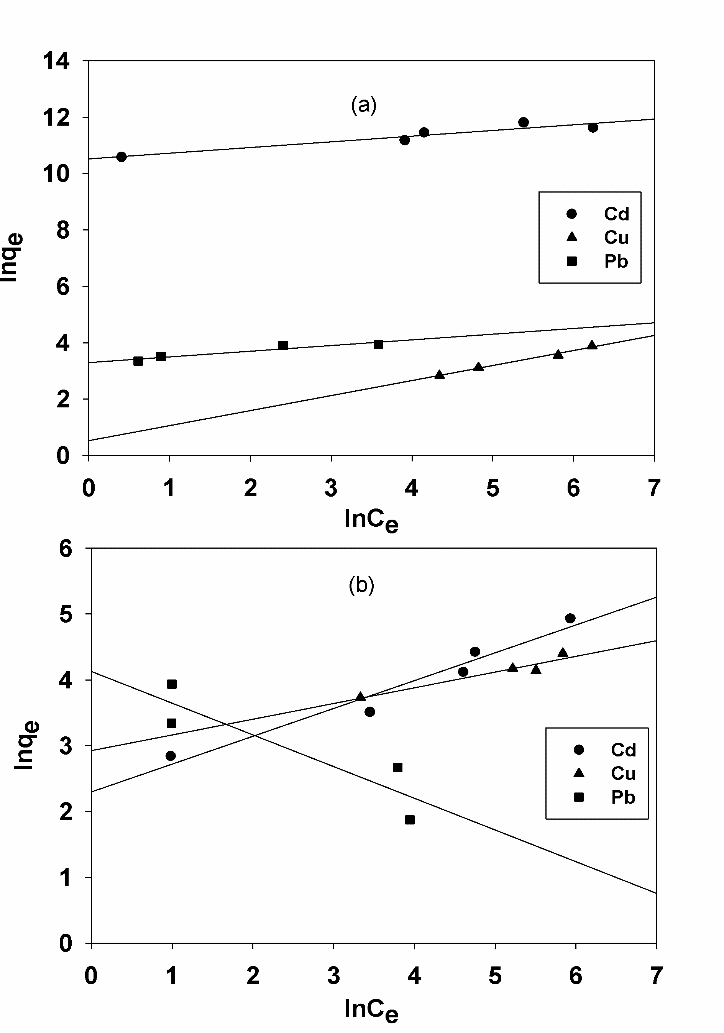
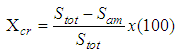
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML