-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Polymer Science
p-ISSN: 2163-1344 e-ISSN: 2163-1352
2015; 5(2): 47-53
doi:10.5923/j.ajps.20150502.03
Intercalated Polyamide-Imide Nanocomposite with Montmorillonite
Habib Saedi
Polymer Research Unit, College of Science, Al Mustansiriya University, Baghdad, Iraq
Correspondence to: Habib Saedi, Polymer Research Unit, College of Science, Al Mustansiriya University, Baghdad, Iraq.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
A poly(amide–imide) (PAI) nanocomposite materials were successfully prepared using a poly(amide–imide) (PAI) matrix and organically Cloisite® 20A as a reinforcing agent. The PAI was synthesized by direct polycondensation reaction of bis(4-4'-carboxyphenyl)-N,N'-pyromellitimide acid (diacid-diimide) and 4-phenylenediamine (p-PDA) using microwave irradiation assistant. The PAI/organoclay nanocomposite films (PAI/NC1 and PAI/NC2) containing different amounts (2.5% and 5.0%) of organoclay were prepared via solution intercalation method. The PAI and its nanostructures were investigated by FT-IR, 1H-NMR and CHN elemental analysis. The X-ray diffraction indicted that in PAI/NC1 and PAI/NC2 intercalated dispersion structure was obtained. The water uptake measurements of organoclay/PAI nanocomposites show a high reducing of their water absorption compared with that for pure PAI.
Keywords: Poly(amide-imide), Direct polycondensation, Composite, X-ray analysis
Cite this paper: Habib Saedi, Intercalated Polyamide-Imide Nanocomposite with Montmorillonite, American Journal of Polymer Science, Vol. 5 No. 2, 2015, pp. 47-53. doi: 10.5923/j.ajps.20150502.03.
Article Outline
1. Introduction
- When two or more phases are mixed together to make a composite, a combination of properties that are not available in any of the individual components is possible. A polymer nanocomposite is a hybrid material consisting of polymer matrix reinforced with nano-scale fillers having at least one dimension in the nanometer range. A relatively small amounts (2-5%) of nanometer sized clay particles in nanocomposites [1]. Because the building blocks of a nanocomposite are nanoscale, they have enormous surface area leading to high interfacial area between filler and matrix. Polymer-clay nanocomposites typically exhibited higher mechanical properties [2-3], improved barrier properties [4], increased dimensional stability [5] and improved flame retradency [6] compared to conventional materials, without significantly raising the density of the compound or reducing light transmission (nanoclays are in the same size range as visible light wavelengths). Montmorillonite has been a filler of choice for most of the studies on polymer nanocomposites [7]. In its pristine form, Montmorillonite is hydrophilic in nature, and this property makes it very difficult to disperse into polymer matrices. The most common strategy to overcome this difficulty is to replace the interlayer clay mineral cations with quaternized ammoniumor [8]. This replacement of inorganic exchange cations by organic onium ions of claymineral serves to match the clay mineral surface polarity with the polarity of the polymer and expands the interlayers of clay mineral, thus facilitating the dispersion of polymer precursors or preformed polymer [9-10]. At present there are four principal methods for producing polymer–layered silicate nanocomposites in situ template synthesis, intercalation of polymer or prepolymer from solution, in situ intercalative polymerization and melt intercalation [11]. Intercalation of a polymer from a solution is a two-stage process in which the polymer replaces an appropriate, previously intercalated solvent. Even though this technique has been mostly used with water soluble polymers, intercalation from non-aqueous solutions has also been reported [12–14].Aromatic polyimides PI are one of the most important classes of high-performance polymers due to their excellent thermal, mechanical, and electrical properties. [15]. One of the most important drawbacks of this class of polymers is their difficulty of processing and infusibility, because of their chain rigidity and strong interchain. To overcome these problems, most polymers structure are modified in two different ways: the syntheses of poly(ester-imide)s with accessible transition temperatures [16-18] and poly(amid-imide)s PIAs with good solubility in a common solvent [19-20], without sacrificing thermal stability [21]. PAI is an amorphous polymer, which is injection molded as a thermoplastic and then post cured at elevated temperature to induce condensation polymerization, which results in a thermoset structure with exceptional high temperature capabilities. The polymer is capable of performing under severe stress conditions at continuous temperatures to 260°C [22]. The objective of this article is to investigate a polyamide-imide and montmorillonite MMT nanocomposite PAIN using a solution technique. Poly(amide-imide) PAI was prepared by direct polycondensation of bis(4-4'-carboxyphenyl)-N,N'-pyromellitimide acid (diacid-diimide) with p-phenylenediamine (p-PDA). Structure and morphology of these PAINs were determined by FT-IR, XRD and water absorption measurements.
2. Experimental
2.1. Materials
- 4-Aminobenzoic acid (p-ABA, Fluka) was recrystallized from ethanol, pyromellitic dianhydride (PMD, Aldrich) was recrystallized from acetic anhydride and N-methylpyrolidone (NMP, Fluka) was dried over molecular sieves before use. 4-phenylenediamine (p-PDA, BDH), triethylbenzylammonium chloride (TEBAC, Fluka), glacial acetic acid (OAcH, Aldrich), were purchased as analytical grade products and used as received. Pyridine (py, Fluka), was dried over solid KOH and fractionally distillated. All organic solvents were analytical grade products and were used as received or purified by distillation before use. Organically-modified Cloisite® 20A supplied by Southern Clay Products Inc., Texas, USA.
2.2. Measurements
- 1H-NMR spectra were obtained on a 500 MHz FT-NMR spectrometer (Bruker Instruments, model Avance-ш 500, Germany) at room temperature using DMSO-d6 as a solvent and TMS as an internal standard. FT-IR spectra were obtained with a JASCO model FT/IR 4200 in the 4000–400 cm-1 range on KBr pellets. The inherent viscosities (ηinh) were determined with a Cannon–Ubbelohde viscometer at 30°C, by using polymer solutions in NMP, at a concentration of 0.5 g/dL. X-ray diffraction (XRD) was performed on Philips X-Pert (Cu-Ka radiation, k = 0.15405 nm). Elemental analysis was performed with a Perkin-Elmer 2400 CHN analyzer.
2.3. Synthesis
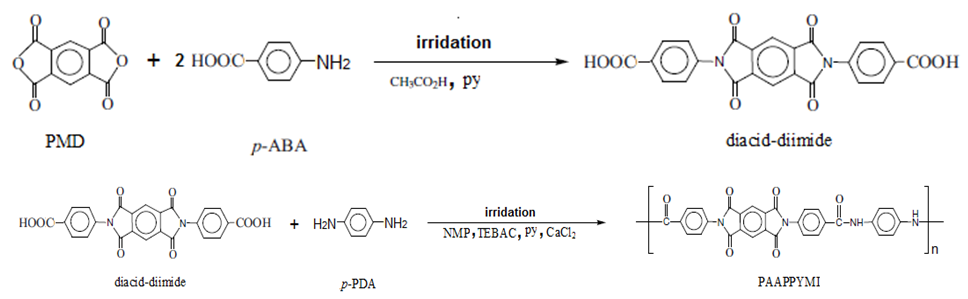
- The polymer poly[(N-4-amino-4-amidoylphenyl) pyromellitimide] PAI was synthesized in two steps from bis(4-4'-carboxyphenyl)-N,N'-pyromellitimide acid (diacid-diimide) and 4-phenylenediamine (p-PDA) (described below and shown in Scheme 1).
2.3.1. Synthesis of bis(4-4'-carboxyphenyl)-N, N'-pyromellitimide acid (diacid-diimide)
- The compound was synthesized by microwave assistant reaction condensation of PMD and p-ABA. Details of the synthesis and characterization data of diacid-diimidehas been described in a preceding article [23].
2.3.2. Synthesis of poly[(N-4-amino-4-amidoylphenyl) pyromellitimide]PAI
- A mixtures of 1.826 g (7.12 mmol) of diacid-diimide, 2 mL of py and 0.8 g of CaCl2 (anhydrous), were located in 100 mL round bottomed flask provided with a magnetic stirrer, overlayered with 10 mL of NMP. The mixture was stirred at ambient temperature until the solution become homogeneous, and then 0.76 g (7.12 mmol) of p-PDA and 1.20 g of TEBAC was added portionwise. The RBF was flashed with argon, equipped with reflux condenser and microwave irradiated (power input: 400 W) for 10 minutes. The reaction mixture was filtered. The filtrate obtained was evaporated under reduced pressure to its half original volume to give a brown viscous which was precipitated by (200 mL) of methanol to give violet-brown precipitate, washed thoroughly with hot methanol and dried under vacuum at 60-70°C overnight. The yield is 74% and ηinh = 0.38 dL/g. For C30H16N4O8, Ncalcd.=9.99%, Nfound. = 10.02%. IR (KBr) v˜ 3347, 1774, 1725, 1647, 1514, 1607, 1515, 1395 and 722 cm-1.1H-NMR (DMSO-d6, δ, ppm): 9.071, 8.384, 8.031-8.002, 7.780-7.763 and 7.487-7.470.
2.3.3. Preparation of PAI/clay Nanocomposite Films PANC1 and PANC2
- The PAI/clay nanocomposite films PA/NC1 and PA/NC2 were prepared via solution intercalation method. An amount of PAI was dissolved in NMP. The organoclay nanoparticles (2.5% and 5%) in NMP were added into the PAI solution to yield particular nanocomposite concentrations of PA/NC1 and PA/NC2. A constant stirring at ambient temperature for overnight was applied to control the dispersibility of organoclay in polyamide matrix. The mixture solution at any concentration was then poured on a petri dish. The films were initially heated to remove the solvent in the vacuum oven. The temperature was controlled as follows: at 60°C for 4 h, at 80°C for 6 h, increased to 120°C for 6 h, and finally, kept at 160°C for 6 h. The experimental details of the PAI/clay nanocomposite film preparation are shown in Scheme 1.
2.4. The Water Absorption of PAI/NC1 and PAI/NC2
- The water absorption of polyamide nanocomposites was determined according to the specifications of ASTM D570–81. In brief, the films (PAINC1 and PAINC2) were dried in a vacuum oven at 80°C to a constant weight and then immediately weighed to get the initial weight (Wo). The films were soaked in deionized water maintained at 25°C. After 24 h, the films were removed from water, quickly placing between sheets of filter paper to remove the inherent water and immediately weighing for any weight gain. This process was repeated till the films attained a constant weight. The total soaking time was 168 h and the samples were weighed at regular 24 h time intervals to get the final weight (Wf). The percentage increase in weight of the samples was calculated by using the formula (Wf-Wo)/Wo.
3. Results and Discussion
3.1. Synthesis of PAI
- This polymer was synthesized through microwave irradiation direct polycondensation of bis(4-4'-carboxyphenyl)-N,N'-pyromellitimide and 4-phenylenediamine using TEBAC as interfacial agent, (Scheme 1).
 | Scheme 1. Route of PAI Synthesis |
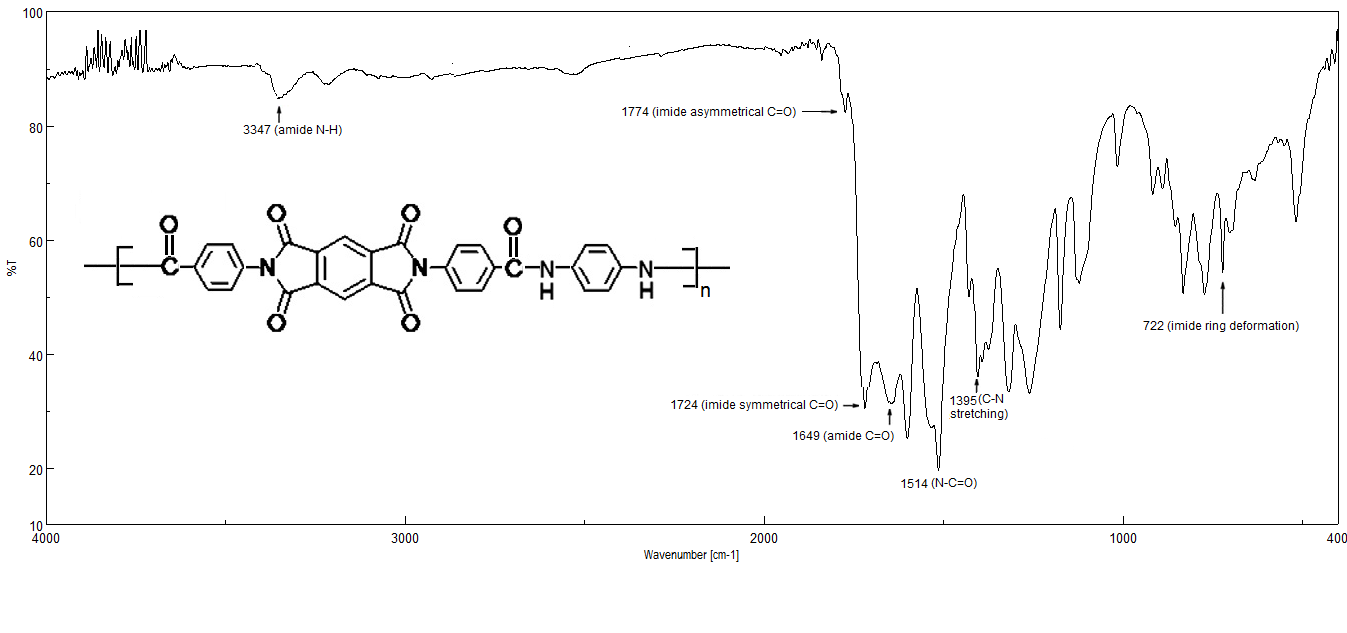 | Figure 1. FT-IR Spectra of PAI |
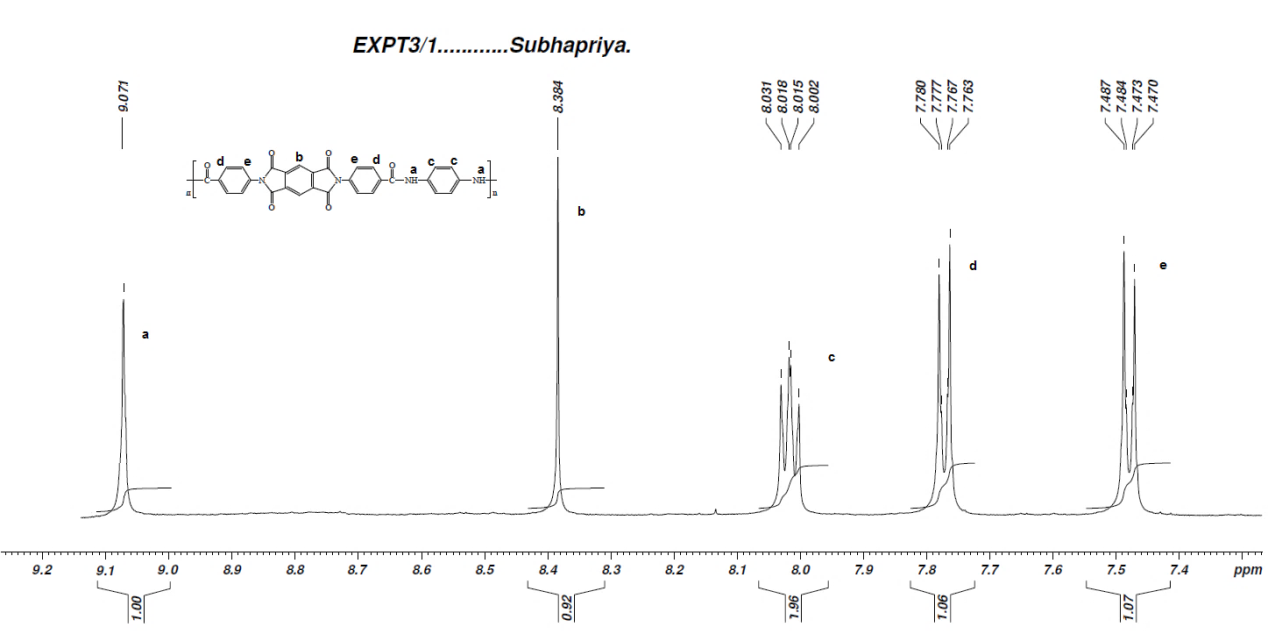 | Figure 2. 1H-FT-NMR spectra for PAI |
3.2. Synthesis of Poly(amide-imide)/ Nanocomposite PAI/NC1 and PAI/NC2
- The thin film obtained from pure PAI was transparent and of slightly brown color in which changed to brown on the addition of organoclay. The transparency of the composite films was decreased gradually with the addition of organoclay, and the films at 5 wt-% organoclay became semitransparent. The characterization of nanocomposites using various techniques is described below.
3.2.1. FT-IR Spectroscopy
- Colisite® consists of organically modified nanometer scale which layered by aluminum and silicate platelets. The replacement of inorganic exchange cations by organic onium ions on surface of clay not only serves to match the clay surface polarity with the polarity of the polymer but also expands the interlayers of clay, thus facilitating the penetration of polymer precursors by solution intercalation method. The FT-IR of AIP/NC2 (Fig. 3) reveals the typical characteristic bands of the PAI polymer matrix, such as the N-H stretching at 3347 cm-1, the amide C=O region at 1640 cm-1, and the bands at 1774 and 1724 cm-1 associated with the imide carbonyl band. The FT-IR spectra also shows the characteristic stretching and bending vibrations of Si-O-Si and Mg-O at 1096 cm-1,460 cm-1respectively [24-25], which improved the interaction between silica nanoparticles and the PAI matrix. All the characteristic peaks in the composite was insensitive to the presence of the silica component, indicating good interaction between the spherical silica and the PAI polymer matrix.
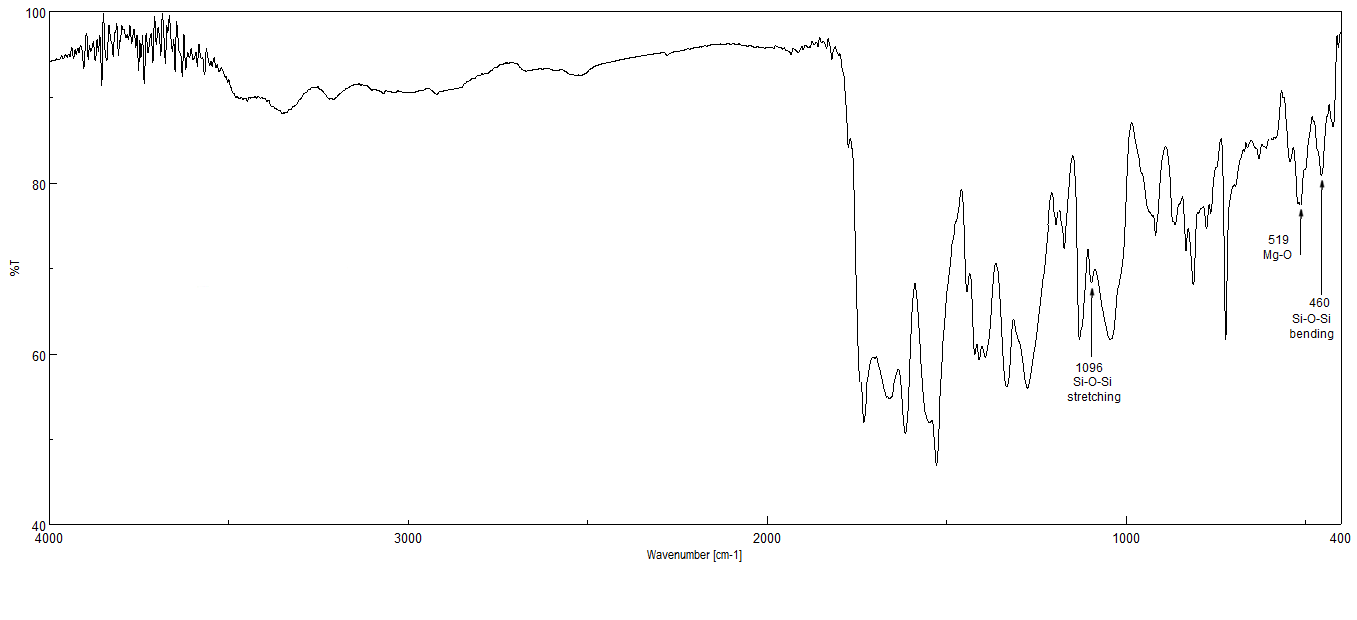 | Figure 3. FT-IR Spectra of PAI/NC2 |
3.2.2. X-Ray Diffraction
- Depending on the nature of the components used and the method of preparation, two main types of composites (intercalated and exfoliated) may be obtained when a layered clay is associated with a polymer. XRD was used to characterize the microstructures of organoclay and different poly(amide-imide)/nanocomposite PAI/NC1 and PAI/NC2 (2.5% and 5.0%) (Fig 4). The XRD pattern shows an increase in d-spacing from 1.79 nm (2θ = 7.496°) for the organoclay to 2.03 nm (2θ = 5.08°) and 2.48 nm (2θ = 3.30°) for organoclay nanocomposites PAI/NC1 and PAI/NC2 respectively. These results indicated that the intercalation of the polymer chains usually increases the interlayer spacing, in comparison with the spacing of the organoclay used, leading to a shift of the diffraction peak towards lower angle values and gives the direct evidence that the synthesized PAI/ organoclay nanocomposites have been formed and show an intercalationdispersion and affects the organoclay dispersion in the polymer matrix.
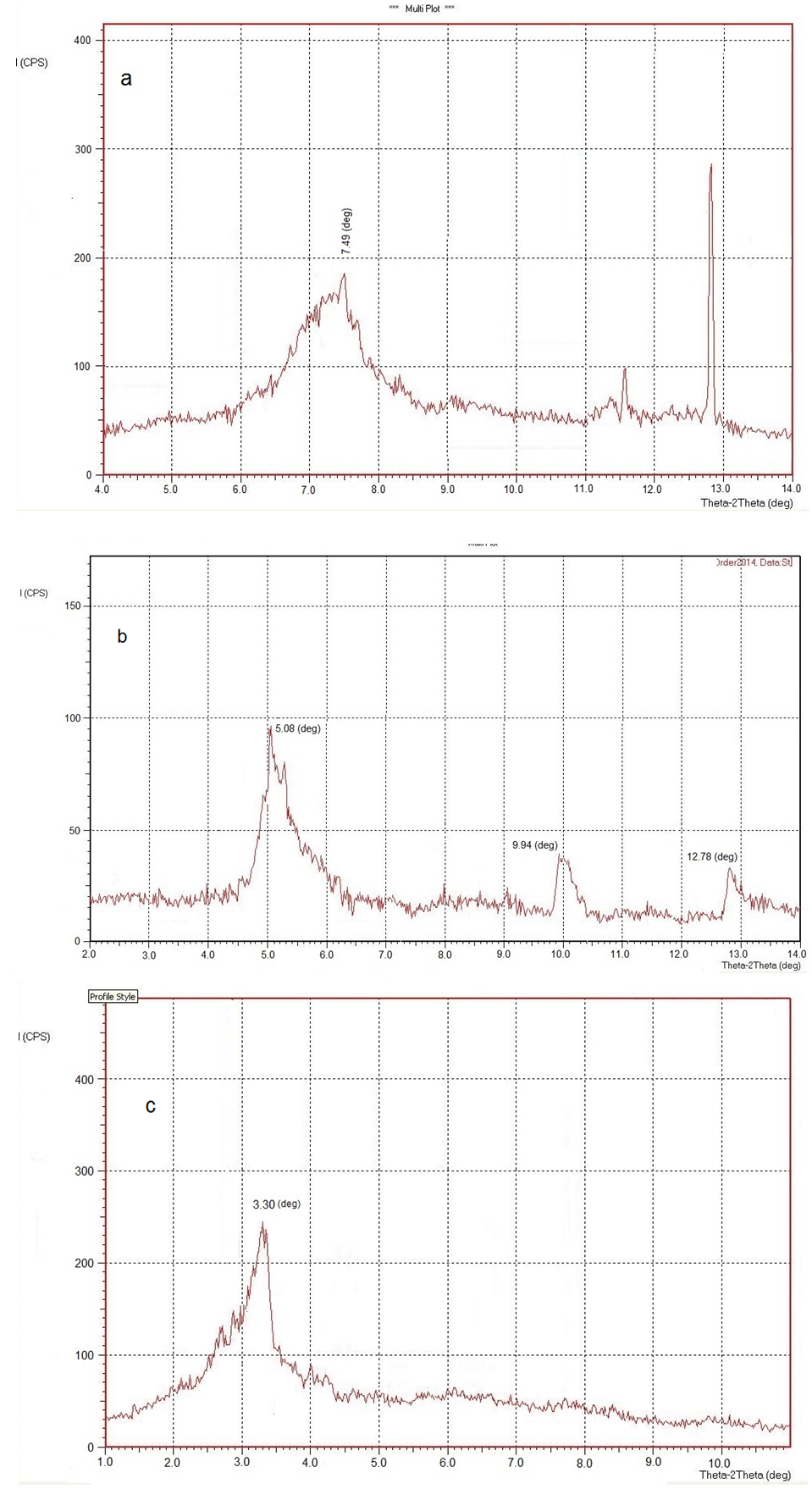 | Figure 4. X-ray patterns of organoclay (a), nanocomposite films PAI/NC1 (b) and PAI/NC2 (c) |
3.3. Water Absorption Measurements
- The presence of polar amid groups in the backbone of the PAI increases its tendency to absorber the water molecules through the hydrogen bonding in which can adversely affect mechanical and dielectric properties [26]. The experimental data were based on the measurement of water absorption of these synthesized PAI/ organoclay nanocomposites under saturated conditions ASTM D570-81 for 168 h. The pure PAI shows 9.83% water absorption but when its intercalated with organoclay the water absorption ratio be reduced to 3.01% and 0.09% at the organoclay concentration 2.5% and 5.0% respectively. The bonding between such polymers and organoclay reduces the validity of amide groups to interact with water.
4. Conclusions
- PAI/clay nanocomposites PA/NC1 and PA/NC2 were prepared successfully through solution intercalation method of PAI with different particular nanocomposite concentrations. The FTIR technique reveals that the silicate layers in the poly(amide-imide)/nanocomposite PAI/NC1 and PAI/NC2 successfully bonded with PAI. XRD patterns show an increasing in the interlayer spacing associated with a shift in the diffraction peak towards lower angle values to gives the direct evidence that the synthesized PAI/ organoclay nanocomposites have been formed as the nature of intercalating agent also affects the organoclay dispersion in the polymer matrix. The water uptake measurements indicated that the presence of low concentration 2.5%-5.0% of organoclay nanocomposite decrease the pure PAI water absorption from 9.83% to 3.01% and 0.09% respectively in which enhanced the mechanical and dielectric properties.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML