-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Polymer Science
p-ISSN: 2163-1344 e-ISSN: 2163-1352
2015; 5(1): 30-34
doi:10.5923/j.ajps.20150501.05
Effect of Halloysite Nanoclay on Polymerization and Properties of Poly(3,4-(2,2-dimethylpropylenedioxy) - thiophene-co-aniline)
Ayesha Kausar
Nanosciences and Catalysis Division, National Centre For Physics, Quaid-i-Azam University Campus, Islamabad, Pakistan
Correspondence to: Ayesha Kausar, Nanosciences and Catalysis Division, National Centre For Physics, Quaid-i-Azam University Campus, Islamabad, Pakistan.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
In the present work, poly(3,4-(2,2-dimethylpropylenedioxy)thiophene-co-aniline) was prepared through in-situ polymerization of 3,4-(2,2-dimethylpropylenedioxy)thiophene and aniline (PDMPT-co-PANI/Halloysite). Later, halloysite nanoclay reinforced PDMPT-co-PANI composites has been prepared and their thermal stability and flammability were investigated. Molecular weight of in-situ polymerized PDMPT-co-PANI copolymer was found to decrease according to gel-permeation chromatography. The PDMPT-co-PANI/Halloysite composites were nanodispersed with layered morphology according to FESEM analysis. They composites exhibited higher heat stability and considerably reduced peak heat release rate with the halloysite nanoclay addition. The reasons were the formation of ceramic char layer over the composite surface during combustion and physico-chemical adsorption of volatile products on silicate surface. The addition of nanoclay formed inorganic-rich surface leading to enhanced thermal stability and reduced flammability of the composites.
Keywords: Halloysite, In-situ polymerization, Gel-permeation chromatography, Thermal stability, Flammability.
Cite this paper: Ayesha Kausar, Effect of Halloysite Nanoclay on Polymerization and Properties of Poly(3,4-(2,2-dimethylpropylenedioxy) - thiophene-co-aniline), American Journal of Polymer Science, Vol. 5 No. 1, 2015, pp. 30-34. doi: 10.5923/j.ajps.20150501.05.
Article Outline
1. Introduction
- In the field of conductive polymers, incessant technological developments have directed towards new applications. A range of conjugated polymers having fine physical properties have been developed. The improved properties of conjugated polymers are their fine processibility, electrical properties, and thermal stability [1]. In order to attain fine material properties molecular structure of organic backbone can be tailored. Structural alterations have rendered these polymers important in sensor protection, optical computing, and optical wave guide devices [2]. One of the methods for improving the processibility of conjugated polymers is the copolymerization with some solvent miscible fragments. The properties of copolymerized material such as toluidine and anisidine depend on the type of substitution. Studies have demonstrated that the electron withdrawing group decreases the electron density on aromatic ring (aniline) while electron donating group increases the electron density on the ring [3]. Consequently, the formation of poly(aniline-co-m-nitroaniline) (a copolymer of aniline with m-nitroaniline) has been reported by chemical and electrochemical polymerization [4].Flame retardancy of polymeric materials has been improved using a number of polymer additives. Usually, conventional fillers are not efficient flame retardants and the physical properties of polymers are inadequately affected by the addition of fillers. Initially, halogenenated (bromine and chlorine), phosphorus-containing, inorganic, and melamine flame retardants have been explored. Among these flame retardants, inorganic fillers are non-hazardous and may not deteriorate the materials properties. Nanoadditives are also an important category of flame retardants [5]. Commonly employed inorganic flame retardants are nanoclay [6], carbon nanotubes [7], graphene [8], and nanosilica [9]. Major roles of the inorganic nanoadditives comprise noteworthy advancement in the physical properties of polymer such as thermal stability, flame retardancy, oxidative stability, etc. When exposed to fire, nanoadditive form superb gas barrier, so deferring the oxidative degradation of resin. Furthermore, the larger surface area of nanoadditives might persuade larger char yield averting the heat exposure. However, the key impediments towards the efficacy of inorganic nanoadditive are the aggregation of nanoparticles and increase of resin viscosity at high loading levels [10-12]. Recently nanoclays have been extensively explored as flame retardants. Nanoclays are found to show decrease in peak heat release rate (PHRR), increase in char residue, and decrease in rate of mass loss during cone calorimetric experiments [13, 14]. Nanoclays also overcome the drawbacks shown by non-silicate additives. According to recent research, flame retardant mechanism involves the formation of carbonaceous silicate char over the composite surface thus insulating the underlying material [15]. Thermal stability and flammability of montmorillonite-based composite systems have been explored extensively [16, 17]. These nanocomposites may have lowest PHRR; although no noticeable decrease in PHRR values were observed [18]. Here we chose to study a copolymeric system of 3,4-(2,2-dimethylpropylene-dioxy)thiophene and aniline for two reasons. First, the thermal stability of rigid-rod materials would be expected high in accordance with polyaniline and polythiophene systems. Secondly, sulfur containing thiophene moiety may also contribute to flame retardance in addition to the nanoclay used.
2. Experimental
2.1. Materials
- Halloysite nanoclay (nanopowder, nanotube diam.×L = 30-70 nm×1-3 μm; pore volume = 1.26-1.34 mL/g), 3,4-(2,2-dimethylpropylenedioxy)thiophene (97 %), aniline (≥ 99.5 %), iron(III) chloride (FeCl3 reagent grade, 97 %), and hydrochloric acid (HCl, 37 %) were obtained from Aldrich.
2.2. Characterization
- The number average and weight-average molecular weight (Mw) were measured using Viscotek TDAmax. Field Emission Scanning Electron Microscopy (FE-SEM) of freeze fractured samples was performed using JSM5910, JEOL Japan. Thermal stability was verified by METTLER TOLEDO TGA/DTA thermo gravimetric analyzer using 3 mg of the sample in Al2O3 crucible at a heating rate of 10 C/min. The combustion properties of nanocomposites were calculated using cone calorimetry. Samples having dimensions 100 × 100 × 5 mm3 were exposed to a FTT 0007 cone calorimeter (FTT Company, England) under a heat flux of 50 kW/m2 according to ISO-5660 standard procedure. Typical results from cone calorimeter reported here were the averages of triplicate.
2.3. Functionalization of Halloysite Nanoclay
- The aminopropyl-functionalized halloysite nanoclay was prepared by adding 3-aminopropyltriethoxysilane (10 mL) to an ethanolic solution of halloysite nanoclay (1 g) in ethanol (20 mL). After 2 h, the slurry obtained was stirred overnight, and then the precipitate was collected after centrifugation. The precipitate was washed with absolute ethanol and dried at 60 °C.
2.4. Preparation of poly(3,4-(2,2-dimethylpropylenoxy) thiophene-co-aniline) (PDMPT-co-PANI)
- For polymerization, 3,4-(2,2-dimethylpropyl- enedioxy) thiophene (0.5 mL) was added in 0.1 M HCl (50 mL) and sonicated for 12 h. To the above solution, 50 mL of FeCl3/HCl solution (1.2 g FeCl3/0.1 M HCl) was added drop wise. Afterwards, a 50 mL solution of aniline (0.5 mL) and 0.1 M HCl was prepared and added slowly to the above mixture. The mixture was further agitated for 12 h resulting in the co-polymerization of aniline and thiophene-based monomer. The sample was filtered, washed (water/ethanol) and dried to obtain PDMPT-co-PANI powder [19].
2.5. Preparation of PDMPT-co-PANI/ Halloysite Composites
- The PDMPT-co-PANI/Halloysite composite, in the proportions of 1, 3, 5 and 10 % of nanoclay were prepared via in-situ polymerization of3,4-(2,2-dimethylpropylene-dioxy)thiophene and aniline. Initially, the solution containing aniline (0.5 mL),3,4-(2,2-dimethylpropylenedioxy)thiophene (0.5 mL) and FeCl3/HCl solution (50 mL) along with the desired amount of clay was refluxed for 24 h at 60 °C. The composite obtained was filtered (cellular membrane filter paper) and washed several times with water and ethanol to remove the residual oxidant. Finally, the product was dried at 60 °C for 6 h. FTIR (KBr, cm-1): 3403 (O−H stretch), 3378 (N−H stretch), 1596 (N−H bend), 2999 (Ar C−H stretch), 2909 (Aliph C−H stretch), 1413 (C−N), 1233 (C−O), 1050 (C−S).
3. Results and Discussion
3.1. Effect of Nanoclay Addition on Molecular Weight
- Gel-permeation chromatography was used to study the effect of nanoclay addition on molecular weight of PDMPT-co-PANI copolymer (Table 1). Addition of nanoclay during the in-situ copolymerization of PDMPT-co-PANI was studied using dilute samples. The presence of filler was expected to decrease the molecular weight of the copolymer. The Mn and Mw of copolymer were found to be 19×103 and 25×103 and gmol-1 respectively. In PDMPT-co-PANI/Halloysite 1 with 1 wt. % nanoclay, the number average and weight average molecular weights were decreased to 18 and 22×103 gmol-1 respectively. For PDMPT-co-PANI/Halloysite 3 having 3 wt. % filler content, the molecular weight values were further decreased to 15 and 19×103 gmol-1 respectively. Similarly, PDMPT-co-PANI/ Halloysite 5 revealed more decline in the property to Mn = 12 gmol-1 and Mw = 17 gmol-1. With 10 wt. % filler content, the number average molecular weight reached the value of 9×103 gmol-1, while the weight average molecular weight decreased to 11×103 gmol-1 in PDMPT-co-PANI/Halloysite 10. Nevertheless, the values were fairly higher to represent reasonable molecular weight. Briefly, the adding up of nanoclay deficiently affected the copolymerization of PDMPT and PANI during in-situ process.
|
3.2. Morphology of PDMPT-co-PANI/ Halloysite Composites
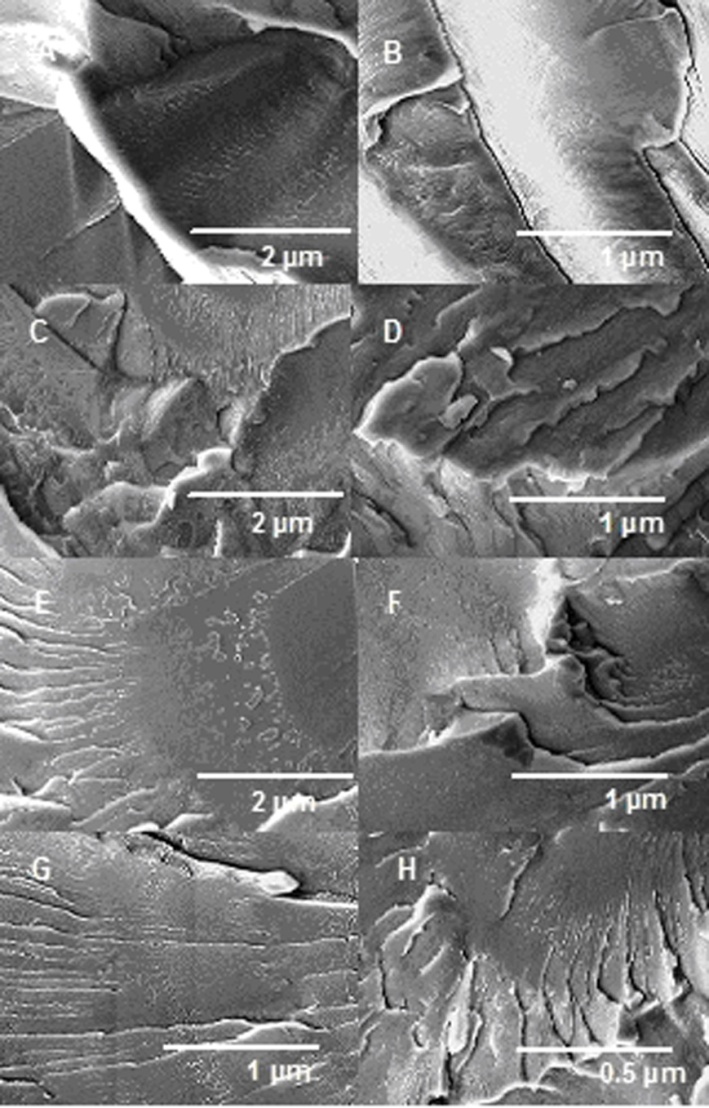
- Fig. 1 shows the dispersion state of composites observed by FESEM images of fractured surfaces of PDMPT-co-PANI/Halloysite. The fractured surfaces of PDMPT-co-PANI/Halloysite 1 (Fig. 1A & B) seem to be smooth and showed some signs of plastic flowing corresponding to brittle failure of homogenous material. Differing from the fractured surface of 1 wt. % loaded clay composite, the fractured surface of PDMPT-co-PANI/ Halloysite 3 revealed indication of less plastic deformation having layered surface and rough fractured pieces (Fig. 1C & D). This type of morphology corresponds to improved toughness owing to the existence of layered silicate structure in matrix. However, the morphology of PDMPT-co-PANI in the presence of 5 wt. % nanoclay was changed. Fig. 1E & F showed relatively fine layering of matrix and nanoclay in PDMPT-co-PANI/Halloysite 5. The morphology has shown that there existed optimal concentration (5 wt. % nanoclay) for better dispersion of nanofiller in the matrix. Similarly, PDMPT-co-PANI/Halloysite 10 has shown uniform layering of the copolymer matrix and uniform dispersion of the clay particles (Fig. 1G & H). With the increasing clay content, PDMPT-co-PANI and halloysite nanoclay layers were found to be closely stacked compared with the 1 wt % of the nanofiller composite.
3.3. Thermogravimetric Analysis
- Thermogravimetric analysis (TGA) was used to examine the effect of halloysite nanoclay addition on the thermal stability of PDMPT-co-PANI copolymer matrix. Fig. 2 shows the weight loss of pristine copolymer, PDMPT-co-PANI/Halloysite 1, PDMPT-co-PANI/ Halloysite 3, and PDMPT-co-PANI/Halloysite 5 composites. Neat copolymer had initial degradation temperature (T0) of 355 °C, 10 % degradation temperature (T10) of 379 °C and maximum degradation temperature (Tmax) of 399 °C respectively. As can be seen from thermograms that there is a clear difference in the thermogravimetric curves of 1 and 10 wt. % nanoclay loaded composites. PDMPT-co-PANI / Halloysite 1 had T0, T10 and Tmax of 420, 432 and 521 °C respectively. PDMPT-co-PANI/Halloysite 5 showed higher values of T0, T10 and Tmax of 468, 479 and 567 °C respectively. The thermal stability of PDMPT-co-PANI / Halloysite 10 was further increased to 587 °C for maximum degradation temperature. The thermal stability of the composites was found to be higher than the literature clay materials [20, 21].
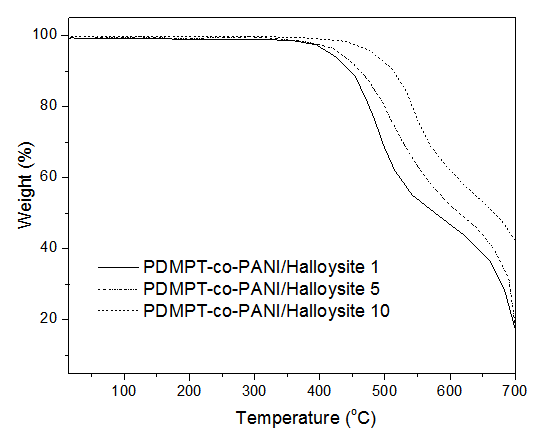 | Figure 2. Thermogravimetric analysis of PDMPT-co-PANI/Halloysite composites |
|
3.4. Flame Retardancy of PDMPT-co-PANI/Halloysite Composites
- In order to investigate the flame retardancy, cone calorimetery technique was used. Fig. 3 shows overlay plots of heat release rate (HRR) versus time for PDMPT-co-PANI/Halloysite composites; the corresponding data are summarized in Table 3. As for PDMPT-co-PANI/Halloysite 1 composite, the maximum peak (PHRR) (370 kW/m2) displays 5 % decrease with respect to PDMPT-co-PANI (390 kW/m2). However, with respect to PDMPT-co-PANI/Halloysite composites, it can be seen that all the composites have almost comparable length of igniting time to peak HRR. PDMPT-co-PANI/Halloysite 3 composite, displayed 13 % reduction (325 kW/m2) in PHRR compared with PDMPT-co-PANI/Halloysite 1. There was further decrease in the peak heat release rate for PDMPT-co-PANI/Halloysite 5 composite (280 kW/m2). PDMPT-co-PANI/Halloysite 10 composite had HRR of 238 kW/m2 i.e. 36 % lower than PDMPT-co-PANI/Halloysite 1. The heat release rate of composites during combustion indicated that the nanoclay has significantly slowed down the combustion process and have shown better flame retardancy. The higher halloysite content was therefore more effective. The flammability of the composites was found comparable to the reported materials [20, 22, 23].
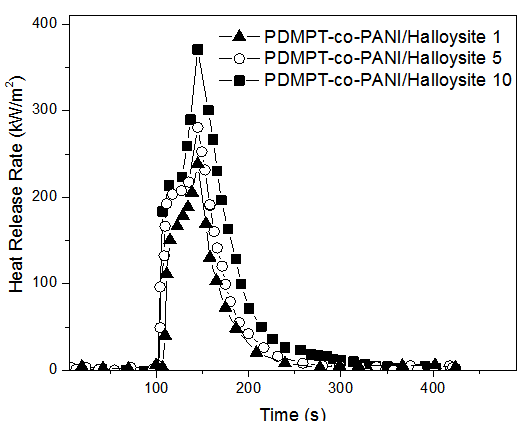 | Figure 3. Dependence of heat release rate on time of PDMPT-co-PANI/Halloysite composites |
|
4. Conclusions
- In this work, a novel copolymer poly(3,4-(2,2-dimethylpropylenedioxy)thiophene-co-aniline) was prepared through in-situ polymerization and reinforced with halloysite nanoclay in various content. Effect of nanoclay addition on the number average and weight average molecular weight of copolymer revealed that the addition of filler hindered the copolymer chain growth. Later the morphology, thermal stability and flammability have been investigated. The addition of halloysite considerably increased the decomposition temperature of the matrix and reduced the PHRR of combustion. This was not only due to the barrier effect, but also because of the physico-chemical adsorption of the volatile degradation products on the nanoclay. TGA thermograms of the composite samples showed that the addition of nanoclay increased the initial and maximum decomposition of PDMPT-co-PANI/Halloysite under nitrogen. The HRR plots showed that the nanoclay inclusion reduced the ignition of copolymer matrix. It was observed that a compact char layer was formed on the surface of PDMPT-co-PANI/Halloysite composite during the combustion test. This was an important factor to increase the flame retardancy of these composites.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML