-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Polymer Science
p-ISSN: 2163-1344 e-ISSN: 2163-1352
2015; 5(1): 18-23
doi:10.5923/j.ajps.20150501.03
Copolymerization of N-vinylpyrrolidone with N,N'-methylen-bis-acrylamide: Properties and Structure
Shamo Tapdiqov, Nizami Zeynalov, Dilshad Babayeva, Elnara Nasiyyati, Saadat Humbatova
Institute of Catalysis and Inorganic Chemistry named after Acad. M. Nagiev, Azerbaijan National Academy of Sciences, Baku, Azerbaijan
Correspondence to: Shamo Tapdiqov, Institute of Catalysis and Inorganic Chemistry named after Acad. M. Nagiev, Azerbaijan National Academy of Sciences, Baku, Azerbaijan.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
The reaction of copolymerization of N-vinylpyrrolidone with N,N'-methylen-bis-acrylamide by mass ratio 1, 2 and 3% with the presence of initiator has been conducted. It is determined that along with cross- linked polymers there are also non-cross linked homo-polymer of poly-N-vinylpyrrolidone and copolymer of N-vinylpyrrolidone with N,N'-methylen-bis-acrylamide are obtained. The structures of obtained products by FTIR and 1H, 13C NMR spectroscopy have been investigated as well.
Keywords: N-vinylpyrrolidone, Copolymerization, Cross-linking hydrogels, FTIR, NMR, Immobilization, Trypsin
Cite this paper: Shamo Tapdiqov, Nizami Zeynalov, Dilshad Babayeva, Elnara Nasiyyati, Saadat Humbatova, Copolymerization of N-vinylpyrrolidone with N,N'-methylen-bis-acrylamide: Properties and Structure, American Journal of Polymer Science, Vol. 5 No. 1, 2015, pp. 18-23. doi: 10.5923/j.ajps.20150501.03.
Article Outline
1. Introduction
- Recently it is very interesting to synthesize gel-forming polymers swelling in water which consider various functional groups [1-3]. Sensitive to pH - condition, temperature, ionic strength, electric field, ultra-violet radiation, such polymeric materials are also called smart or intelligent materials [4-6]. Such systems sharply changing the volume of interactions of the specified environments and result these polymers are widely used in medicine and biotechnology for purify biologically active compounds and also an immobilization of biocatalysts [7-9].Synthesis of hydrogels on basis of synthetic polymers and immobilization on the biologically active compounds for the purpose of receiving complex compounds are in attention of many researchers today [10]. Ability of hydrogels to absorb a great amount of water is to give immobilization to antibiotics, enzymes, alkaloids and other biologically active compounds [11].To regulate separation speed of biologically active compounds it is possible to change parameters of the - pH, temperature, radiation or change the molar ratio of monomer and cross-linked agent in the processes of gel synthesis [12, 13].In this case, biologically active compounds don't have chemical bond with hydrogel and its separation depends on hydrogel and its structure [14]. The main demand made to hydrogels, used for the purpose of transportation of biologically active agents is a regulation of extent of their swelling degree from the irrespective of environment pH [15-17].With the purpose of this scientific research we study to synthesize the three-dimensional polymeric hydrogels received by copolymerization of N-vinylpyrrolidone (VPr) with various molar ratio of N, N'-methylene-bis-acrylamide.We studied the influence of parameters the yield of the obtained products, to investigate hydrogels properties and structure and to check transportation possibility of trypsin as well.
2. Experimental Part
2.1. Materials
- VPr and N, N'-methylene-bis-acrylamide (MBAA) were purchased from Sigma Aldrich. Azobisisobutyronitrile (AIBN) (E. Merck) was purified by recrystallization twice from methanol and then dried in the dark (m.p. 104℃). For the preparation of mixture solution, deionized water was used and we used acetone for precipitation of poly-N-vinylpyrrolidone (PVPr) from Aldrich, too. The other reagents were used as obtained and the solvents were purified according to conventional methods.
2.2. Copolymerization Procedure
- Copolymerization VPr with MBAA for to synthesizing cross-linked polymer has happened by the following method: At first prepared the solution of monomer with MBAA in 1, 2 and 3% mass quantity and mixture till formation of homogonous solution. Add in system as initiator AIBN. Initiator's concentration forming 4.5×10-3 mol/L. After full dissolution of the initiator, to avoid destruction process of polymer by air oxygen, the reactor was vacuum 5 mm. Hg. The copolymerization reaction was taken in 2 hours, at the 323 K. The obtained fractions were separated with acetone and washing in deionized water. The yield of obtained fractional are defined by a gravimetric method. For studying yield of products depend on the time of copolymerization reaction was taken (VPr:MBAA=44:1, CInit.= 4.5×10-3 mol/L, T=343 K) in during 2,5; 5; 10; 15; 20; 60 and 120 minutes. For the purpose of studying the influence of temperature on yield of hydrogel copolymerization reaction taken at a 323; 343; 363 and 383 K temperature. In this process the molar ratio monomer and cross-linking agent are VPr:MBAA=44:1, the initiator concentration 4.5×10-3 mol/L and reaction was taken during 60 minute.
2.3. Measurements and Characterization
- After a copolymerization the structures of the hydrogels and polymers were identified by FTIR and 1H, 13C of a nuclear magnetic resonance (NMR) spectroscopy. The FTIR spectra of the prepared monomer and its polymer were recorded by IR spectroscopy (Varian). 1H NMR spectra were recorded in dimethyl sulfoxide (DMSO) for monomer and polymer with tetramethylsilane as internal standard on Bruker NMR spectrometer. 1H NMR spectra were run at 300 MHz, whereas 13C NMR spectra were recorded in DMSO for monomer and were run at 125 MHz.
3. Results and Discussion
3.1. Effect of the Monomer Concentration on Yield of Copolymerization Products
- Copolymerization reaction of VPr with MBAA is carried out 1, 2 and 3% a mass ratio with participation of the initiator. It is revealed that at allocation of reaction products and research of their structure definite that there are homo-polymer of VPr, copolymer VPr with MBAA and cross-linked polymer among the products. Yields of products in copolymerization reaction are given in table 1.
|
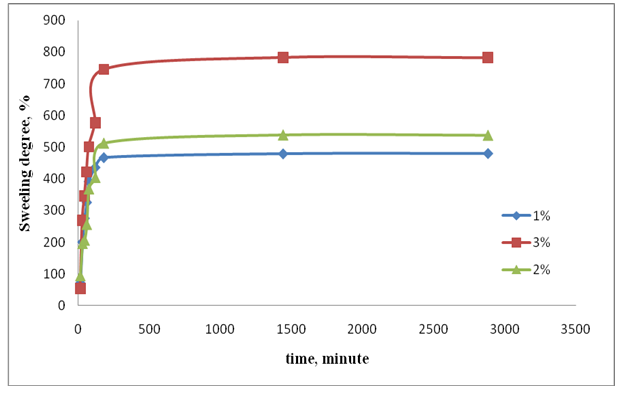 | Figure 1. The swelling kinetics of basis VPr-MBAA gels in 0.9% NaCl solution. T=293 K |
3.2. Effect of the Initiator Concentration on the Rate of Copolymerization
- The influence of initiator's concentration yield of gel fraction in system with the highest transformation of VPr participation with 3% (mass) of MBAA. At the result, it is observed increase of the gel fraction and decrease yield of copolymer fraction. Polymerization reaction direct towards to homo-polymer and forming gel process when increased the concentration of initiator and result yield of their fractions respectively increased. Although increased concentration of the initiator the yield of gel fraction increased but decreased the stability and swelling degree of cross-linking polymer, therefore the concentration of the initiator of 4.5 mmol/L is accepted the optimum. As with increases the concentration of the initiator the molecular mass of the polymer decreases and the cross-linking polymer consist a low molecular weight macromolecules. It was the cause of the little swelling degree which connected with the size of polymeric net.
3.3. Effect of Copolymerization Time
- For the research dependence of yield of cross-linking polymer on time, reaction of copolymerization VPr with MBAA the molar ratio was 44:1, concentration the initiator 4.5×10-3 in mol/L and copolymerization become during 2.5; 5; 10; 15; 20; 60 and 120 minutes. It was shown that, the yield of gel fraction within 60 minutes makes 48.64%. During the reaction it was observed that the yield of copolymer and polymer fractions decreases and a more amount of monomer consumption to the gel-forming process. It was determined that in 60-70 minutes the yield of cross-linking polymers is stabilized (Fig.2) and the gradient of time doesn't impact the gels yield.
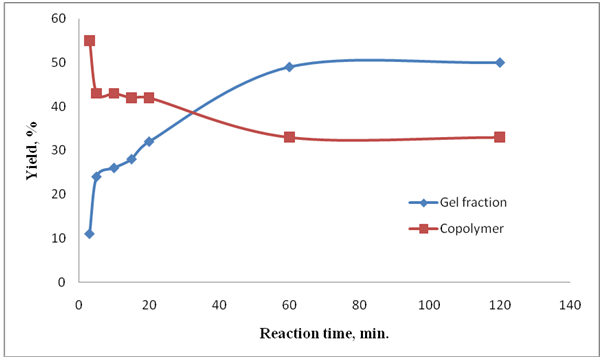 | Figure 2. Depend of yield of cross-linking polymer and copolymer fraction on the reaction time |
3.4. Effect of Temperature
- The influence of the temperature on a gel yield was studied. Experiments were for this purpose made under the above described optimum conditions of a cross-linking (VPr:MBAA=44:1 molar ratio, CInit.=4.5×10-3 mol/L, t=60 minutes). After completion of process all fractions were separated and the yield of products was determined by a gravimetric method (Fig.3).
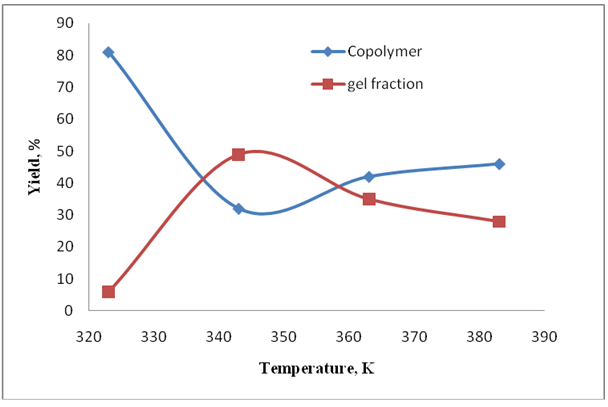 | Figure 3. Yield of gel fraction and copolymer in different temperature |
3.5. Characterization
- The FTIR spectrum of cross-linking gel absorption bands at 1420–1410, 1650 and 1580-1475 cm-1 represent the CH2=CH-, >C=O and -NH- groups (Fig.4). This shows that the double CH2=CH- bands at MBAA don't complete during the copolymerization reaction.
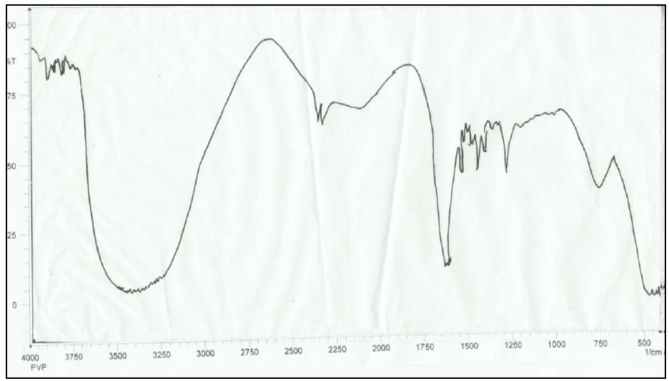 | Figure 4. The FTIR spectrum of PVPr (separated from the copolymers products) |
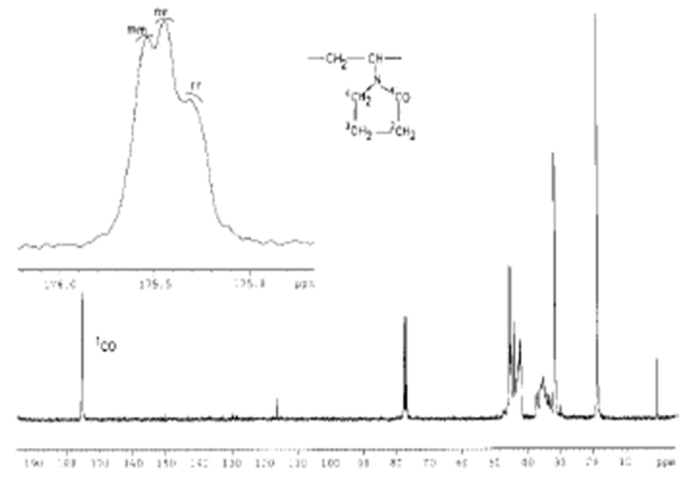 | Figure 5. The NMR spectrum of pure PVPr (separated from the copolymers products) |
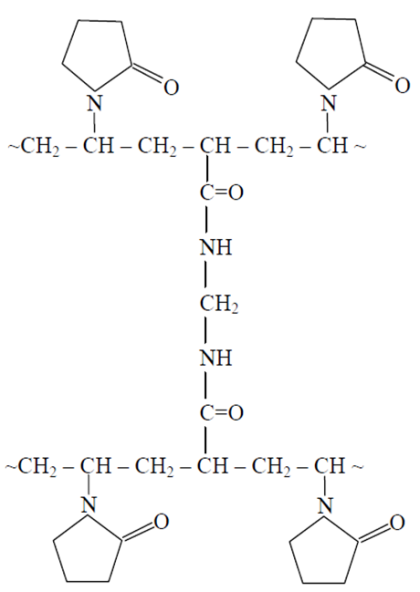 | Figure 6. Schematic illustration of gel fraction based of VPr-MBAA |
4. Conclusions
- It was shown that the materials of three-dimensional structure obtained by copolymerization of VPr with MBAA, possess ability of gel forming in water and they meet the requirements, shown to transportation of biologically active compounds.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML