-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Polymer Science
p-ISSN: 2163-1344 e-ISSN: 2163-1352
2014; 4(5): 123-129
doi:10.5923/j.ajps.20140405.01
Synthesis, Characterizations and Polymerization of Novel Cyano Acrylamide Derivative
S. M. Mokhtar1, Maher Z. Elsabee2, S. M. Abd-Elaziz1, F. A. Gomaa1
1Chemistry Department, Faculty of Women Ain-Shams University, Cairo, Egypt
2Chemistry Department, Faculty of Science, Cairo University, Cairo, Egypt
Correspondence to: Maher Z. Elsabee, Chemistry Department, Faculty of Science, Cairo University, Cairo, Egypt.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Novel N-[4- (4`-cyanophenoxy) phenyl] acrylamide (CPAM) has been synthesized. Its structure has been elucidated by elemental analysis, FTIR and 1H NMR as well as 13C NMR. Free radical- initiated polymerization of CPAM was carried out in THF solution using azobisisobutyronitrile as initiator. The effect of monomer and initiator concentrations and reaction temperature on the rate of polymerization (Rp) was studied. The activation energy of the polymerization was calculated (ΔE= 21.1 kJ/mol). The polymer structure was investigated by FTIR and 1H NMR. The properties of the prepared polymer, including thermal behavior, thermal stability, solubility and solution viscosity, were studied.
Keywords: Benzonitrile, Acrylamide, Free- radical polymerization, Kinetics, FTIR, NMR, Thermal analysis
Cite this paper: S. M. Mokhtar, Maher Z. Elsabee, S. M. Abd-Elaziz, F. A. Gomaa, Synthesis, Characterizations and Polymerization of Novel Cyano Acrylamide Derivative, American Journal of Polymer Science, Vol. 4 No. 5, 2014, pp. 123-129. doi: 10.5923/j.ajps.20140405.01.
Article Outline
1. Introduction
- Polymers containing polar substituent such as cyano groups are of interest in the development of advanced electrical and optical materials because of the large dipole moment arising from their polar substituent (CN) [1]. It has been recognized that the incorporation of aryl-ether linkages generally imparts an enhanced solubility, process ability and toughness of aromatic polyamides and polyimides as well as their copolymers without substantial diminution of thermal properties [2-9]. It appears that polymers having ether, benzonitrile and amide/amide–imide groups within the backbone have not been studied in details. These polymers are expected to have the advantages of both polyether nitrile and polyamide/poly (amide–imide)s in their properties such as high Tg, high temperature resistance, toughness, good process ability and solvent resistance. In addition, the pendant nitrile group appears to promote adhesion of the polymer to many substrates [10, 11] possibly through polar interaction with other functional groups, and it serves as a potential site for polymer cross-linking [12, 13]. N-substituted acrylates are very interesting monomers because they have acquired prime importance in various avenues of applications. Recently acrylamide derivatives were found to have antiviral activity as inhibitors of hepatitis B virus replication [14]. Acrylamide is an important commodity chemical used as coagulators, soil conditioners, stock additives for paper treatment and in leather and textile industry [15]. The polymer of acrylamide is used as flocculent for fine solids suspended in water, pigment retention aid in papermaking [16].Moreover, the N-substituted acrylamides are used to prepare thermo sensitive materials. These thermoplastic polymers present also great potential in application as drug delivery system [17], as glycogen phosphorylase inhibitors [18], human gene vectors and biocatalysts [19]. It is possible to obtain N-acryloyl and N-methacryloyl derivatives of human serum albumin (HSA), in which acryloyl fragments are bound to asparagines and lyzin fragments [20]. The development of antimicrobial macromolecules holds a good promise for novel therapeutics and new materials to prevent the spread of infectious disease [21]. The reaction of acryloyl chloride or methylacryloyl chloride with the corresponding amines to prepare new functional monomers has been reported [21-25].The present work deals with the preparation of a novel acrylamide derivative which contains CN moiety and is readily polymerized by free- radical polymerization. The structure and thermal properties of the monomer, and polymer were studied.
2. Experimental
2.1. Materials
- High purity 4-chloro benzonitrile (CBN) (Aldrich), 4-aminophenol (Aldrich), N- methyl-2- pyrrolidine (NMP) (Aldrich), triethylamine (Aldrich) and acryloyl chloride (E. Merck) were used as received. Azobisisobutyronitrile (AIBN) (E. Merck) was purified by recrystallization twice from methanol and then dried in the dark (m.p. 104℃). The other reagents were used as received and the solvents were purified according to conventional methods.
2.2. Measurements and Characterization
- Standard techniques of characterization such as spectroscopic analyses (FTIR and NMR), X-ray diffraction, thermogravimetric analysis, and differential scanning calorimetric analysis together with solubility and viscosity measurements were investigated. The FTIR spectra of the prepared monomer and its polymer were recorded by IR spectroscopy (4100 JASCO FT/IR). 1HNMR spectra were recorded in DMSO for monomer and polymer with tetramethylsilane as internal standard on Varian Mercury VX-300 NMR spectrometer. 1H NMR spectra were run at 300 MHz, whereas 13C NMR spectra were recorded in DMSO for monomer on JEOL - ECA-500. 13C NMR spectra were run at 125 MHz.The X-ray analysis was carried out using X-ray diffraction instrument Philips Pw 1390 channel control. Cu Target Kα = 1.542, Ni filter is 40 Kv, 25 mA. The Viscosity measurements were carried out using an Ubbelohde suspended level dilution viscometer. DMF was used as solvent with a flow time of 98 seconds at 25℃. The thermogravimetric analysis of the powder was carried out in nitrogen atmosphere with a heating rate of 10˚C min-1 from room temperature up to 800℃ by Shimadzu TGA – 50 H. The glass transition (Tg) temperature was investigated by differential scanning calorimeter analysis (DSC) using Shimadzu DSC - 60 with a heating rate of 10℃ /min under nitrogen atmosphere.
2.3. Monomer Synthesis
2.3.1. Preparation of 4-(4`- amino phenoxy) benzonitrile [8]
- 4-Aminophenol (10 g; 0.0917mol), N- methyl-2- pyrrolidine (70 ml), and toluene (63 ml) were charged into a three-necked flask equipped with a Dean–Stark trap. Powdered anhydrous K2CO3 (18 g; 0.13 mol) was added and water was removed by azeotropic distillation with toluene by refluxing the mixture with stirring under nitrogen atmosphere at 140–150oC. After complete dehydration (3 h), the remaining toluene was removed until the flask temperature reached 160℃. Heating was discontinued and the system cooled to 130℃, then solid 4- chloro benzonitrile (12.608 g; 0.0917 mol) was added in portions and the mixture was stirred at 195–200℃ for 8 h. The mixture was allowed to cool and subsequently added a large excess of water to precipitate the product, which was collected by filtration and dried under vacuum. The product was recrystallized from ethanol/water mixture to obtain pinkish brown solid; yield 80%, melting temperature: 105℃, (molecular weight = 210. 23). The structure of the product was confirmed by elemental analyses at the Micro-Analytical Unit, Cairo University with the following data: Elemental analyses calculated for C13H10N2O: C, 74.27%; H, 4.79%; N, 13.33%. Found: C, 74.29%; H, 4.41%; N, 13.57%.FTIR (KBr) 3327 cm-1, 3222 cm-1 (s,NH2); 2227.38 cm-1 (vs, sharp C ≡ N); 1 240 cm-1 (vs, sharp, phenyl ether). Preparation of N-[4- (4`-cyanophenoxy) phenyl] acrylamide (CPAM) 4, 4`- amino phenoxy benzonitrile 5g (0.0237mole) was dissolved in acetonitrile. In the same solvent acryloyl chloride 2.15g (0.0237mole) was added drop wise. The mixture was kept at low temperature at about -10℃ with stirring by using ice bath. Additionally, to neutralize the hydrochloric acid that is formed as a by- product of the reaction, triethylamine was added throw the funnel. The resulting product was poured into water, filtered, washed with water and dried in an air oven at 60℃. The acrylamide derivative is immediately separated as a pale pink crystals with 80% yield and m.p. = 142℃. The Crude product was then recrystallized twice from ethanol/water. Elemental Analayses: Calculated for C16 H12 N2 O2: C, 72.73; H, 4.5; N, 10.6. Found: C, 72.94; H, 4.22; N, 10.32%. FTIR ( KBr ): v (3425 cm-1- 3298 cm-1) NH, v 1659 cm-1 (CO), v 1238 cm-1 (ph-O- ph), v 2216 cm-1 (C ≡ N), 1532 cm-1 (C=C), 983 cm-1, 842 cm-1. 1H NMR (DMSO - D2O) 7.84-7.807 (d, 2H), 7.807- 7.772 (d, 2H), 7.148- 7.11 ( d,2H), 7.141-7.093 ( d,2H), 6.48 (d, 2H), 5.78 (d, H), 10.26 ( s, NH ).13C NMR (DMSO): 163.6 (CO), 162.08, 150.2 (COC), 105.2 (CCN), 118.07 (CN), 121.6 (4CH, Ar), 136.8 (2CH, Ar), 135.1 (2CH, Ar), 132.2 (CH), 127.5 (CH2), 40.2-39.9.
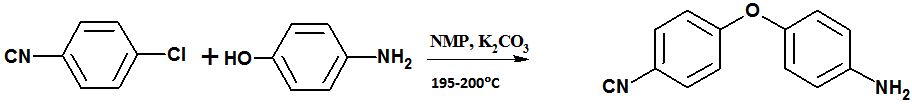 | Scheme 1. Preparation of 4,4`amino phenoxy benzonitrile |
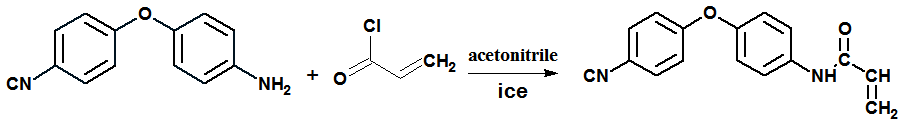 | Scheme 2. Preparation of 4,4`cyano phenoxy phenyl acrylamide (CPPAM) |
2.4. Polymerization Procedure
- Polymerization was carried out in calibrated dilatometers (3-5 ml in capacity) with ground joint stoppers. The % conversion was determined gravimetrically. The polymers were usually purified by re-precipitation from ethanol solution into THF. The rates of polymerization were determined from the slope of the linear concentration vs. time plots.
2.5. Characterization of Poly-CPAM
- IR (KBr): v 3331 cm-1 (NH), v 225 cm-1 (C ≡ N) , v 1240 cm-1 (Ph – O- Ph), 2927 cm-1, 835 cm-1 (CH). 1H NMR (DMSO - D2O) δ 7.67 (d, 2H), δ 7.55 (d, 2H), δ 6.88 ( d,2H), δ 3.4 (d, 2H), δ 2.49 (2H), δ 9.81 (s, NH ), 1.82 (-CH2).
3. Results and Discussion
- Due to the importance and many applications of the compounds cantaining phenoxy, nitrile and acrylamide group, the novel monomer of N-[4- (4'-cyanophenoxy) phenyl] acrylamide (CPAM) was prepared and its structure was confirmed by elementatal and spectral analyses according to the experimental data detail. The polymerization of the prepared monomer was carried out in presence of AIBN as initiator in THF solution and the polymerization reaction proceeded homogenously. The polymerization process was confirmed by spectral tools.The FTIR spectra of poly(cyanopheoxy- phenylacrylamide) (poly-CPAM) supported the polymerization process. The disappearance of the bands at 1532 and 981 cm-1 which are due to the stv. C=C, indicates that the double bond disappeared and the polymerization occurred by the opening of the C=C at acrylamide group. The same observation can be found in the 1H-NMR of the monomer and its polymer. The 1H NMR spectrum of the monomer indicates that the structure of this monomer revealed two olefinic protons which are considerably shifted downfield to appear in the aromatic region and it is hardly distinguished from those of the aromatic protons (= 7.05- 7.84 ppm, 8 H). The 1H NMR of the polymer represents multiple signal at = 1.82 ppm. This indicates the formation of the saturated bond (Fig.1).
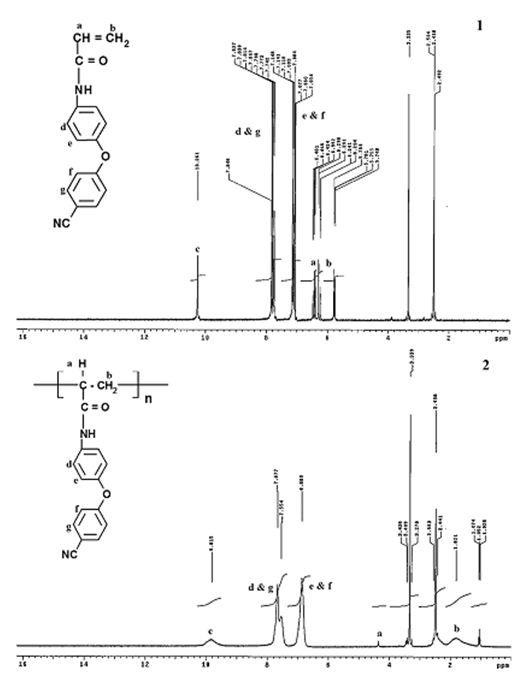 | Figure 1. 1HNMR spectra of: CPAM(1) and Poly-CPAM(2) |
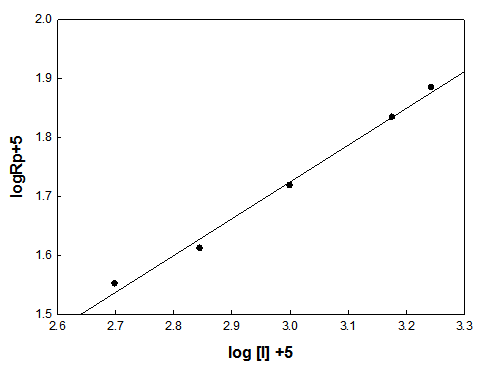
3.1. Effect of the Initiator Concentration on the Rate of Polymerization
- The effect of the initiator concentration on the rate of free radical polymerization was studied at fixed monomer concentration of 0.6 mol/L at 65℃. The concentration of the initiator was varied and log rate of polymerization was plotted vs. log initiator concentration. A straight line was obtained as shown in Fig. (2) The slope of the line gives the kinetic order with respect to the initiator and is equal to 0.57. From this value, the rate of polymerization is proportional with the limits of error to initiator concentration [I] 0.5. This means that the dependence of Rp on [I] is a direct consequence of the bimolecular termination mechanism.
 | Figure 2. Effect of initiator concentration on the rate of polymerization in THF, the monomer concentration [M] = 0.6 mol.L-1 and at 65°C |
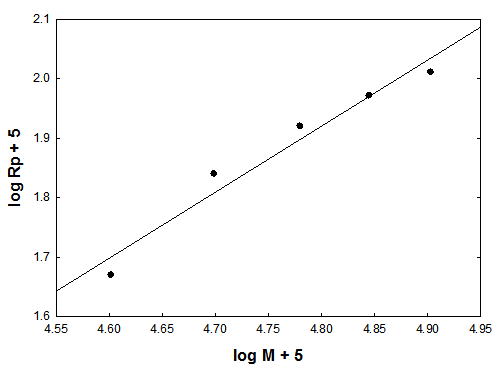
3.2. Effect of the Monomer Concentration on Rate of Polymerization
- The dependence of the initial rates of polymerization (Rp) on the monomer concentration is shown in Fig. (3), which is a log plot of the rate vs. monomer concentration at constant initiator concentration [I], 1 x10-2 mol/ L, and at a temperature of 65℃. The rate of polymerization Rp has been determined from the slope of the change in volume (v) vs. time. The slope of the log plot of the rate vs. monomer concentration equals to 1.1. This value represents the order of the reaction regarding the monomer concentration. It is a little bit higher than the order of magnitude which is unity characteristic to free radical polymerization. This may be attributed to steric and solvent effects. Finally, the equation of the steady state of polymerization can be represented as:
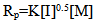 | (1) |
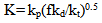 | (2) |
 | Figure 3. Effect of monomer concentration on the rate of polymerization in THF, the initiator concentration [I] =1×10-2 mol. L-1 and at 65 °C |
3.3. Effect of Temperature
- The effect of temperature on the rate and degree of polymerization is of prime importance in determining the manner of performing a polymerization. Increasing the reaction temperature usually increases the polymerization rate and decreases the polymer molecular weight. Fig. (4) shows the effect of temperature on the rate of polymerization. The activation energy of the polymerization can be calculated based on Arrhenius –type equation,
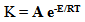 | (3) |
 | Figure 4. Effect of temperature on the rate of polymerization in THF, [M] = 0.6 mol. L-1 and [I]= 1 ×10-2 mol. L-1 |
3.4. Molecular Weight Determination
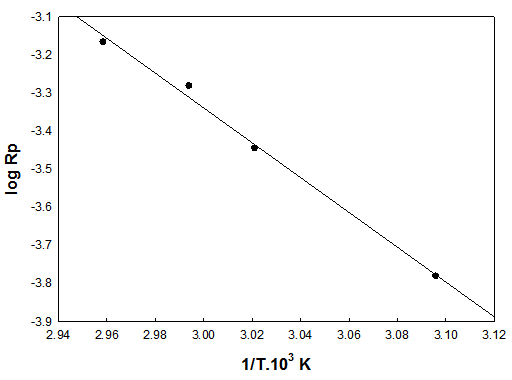
- The intrinsic viscosity of the prepared polymer was measured in DMF at 25℃ using Ubbelohde viscometer. Fig. (5) shows that, the intrinsic viscosity [η] increases with increasing monomer concentration and decreases with increasing the initiator concentration, which is a well expected behavior of addition polymerization mechanisms.
 | Figure 5. Effect of: (a) monomer concentrations and (b) initiator concentrations on the intrinsic viscosity |
3.5. Characterization
3.5.1. X-Ray Diffraction
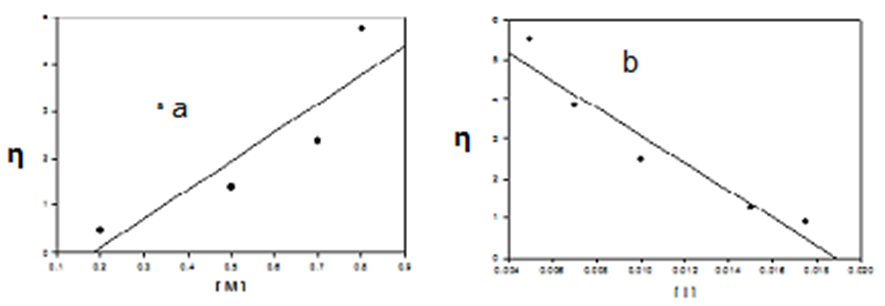
- The x-ray method allows the calculation of the relative amounts of crystalline and amorphous nature in a polymer. The crystallinity of CPAM and its polymer was examined by X-ray diffraction as shown in Fig. (6). The Figure indicates that the monomer structure is highly crystalline, while, poly-CPAM seems to be semi crystalline, where the tendency towards crystalline order is observed with sharp and diffused pattern peaks. This may be attributed to the symmetrical nature of the polymeric chain.
 | Figure 6. The x-ray diffraction of: CPAM monomer (a) and Poly-CPAM (b) |
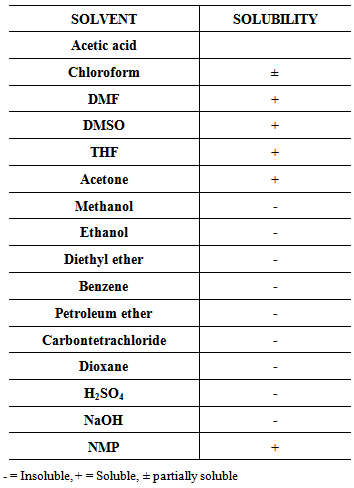
3.5.2. Solubility
- Table (1) shows the solubility of the new poly-CPAM towards some organic solvents and organic and inorganic acids. The data indicates that poly-CPAM is soluble in some polar organic solvents. The solubility of Poly-CPAM could be attributed to the presence of bulky pendant groups, which led to disturbing the regularity and crystallinity of molecular chains and increased the free volume. The resistance of the new polymer to other solvents may be due to the nature of the cyano group [27].
|
3.5.3. Thermal Properties
- Thermogravimetric analysis (TGA) is used to characterize a wide variety of the thermal properties of polymeric materials. The thermal stability of Poly-CPAM was determined by TGA. The weight loss percent curve and DTGA of poly-CPAM occurred via a three stages are shown in Fig. (7). The TGA curve of poly-CPAM shows that the first stage at 49.24℃ is due to evaporation of the residual solvent, after that the polymer decomposition commenced at 300oC and maximum in the DTGA at 390℃ till it reached the end of the major decomposition step at 403℃. The third stage of weight loss at 550℃ to 800℃, peaking at 733℃. The data indicates that the poly-CPAM has high thermal stability as well as the other phenoxy and cyano polymers.
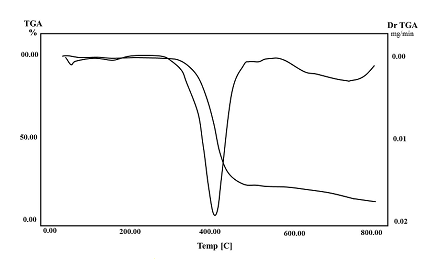 | Figure 7. Thermogravimertic and deferential thermogravemitric analysis of Poly-CPAM |
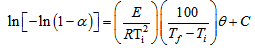 | (4) |
3.5.4. Photo Stability
- A sample of poly- CPAM was exposed to the UV Lamp for an interval time of 10 days. The polymer did not show any change in appearance or weight loss when it was exposed to the UV Lamp of long and short waves. The FTIR spectrum of the exposed sample is shown in Fig. (8), the spectrum showed no changes in the position of the characteristic peaks. This means that the structure of the treated sample did not change. Poly-CPAM showed a good photo-stability besides the thermal stability.
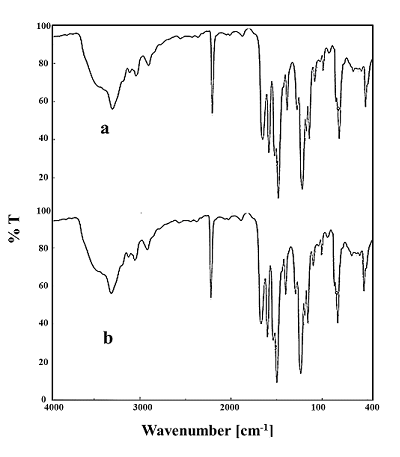 | Figure 8. FTIR spectra of: (a) Poly-CPAM and (b) Exposed Poly-CPAM to a UV Lamp for 10 days |
4. Conclusions
- The new 4, 4` cyano phenoxy phenyl acrylamide (CPAM) monomer was synthesized and characterized. The free radical polymerization of CPAM was initiated by AIBN in THF. The characterization of the monomer as well as the polymer was studied. The kinetics of the polymerization was investigated. The polymer shows a high glass transition temperature (353.5℃) and high thermal stability and photo stability. Also, the polymer exhibits a good solubility in many polar solvents. Further studies are in progress.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML