-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Polymer Science
p-ISSN: 2163-1344 e-ISSN: 2163-1352
2014; 4(4): 107-116
doi:10.5923/j.ajps.20140404.02
Effect of Dodecyl Benzene Sulphonic Acid on the Electrical Conductivity Behaviour of Poly(2-chloroaniline) and Poly(2-chloroaniline)/Silk Blends
Porselvi Linganathan, Jhancy Mary Samuel
Department of Chemistry, Auxilium College, Vellore, Tamilnadu, India
Correspondence to: Jhancy Mary Samuel, Department of Chemistry, Auxilium College, Vellore, Tamilnadu, India.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
The poly(2-chloroaniline) and poly(2-chloroaniline)-DBSA/Silk and P2ClAn/Silk were prepared by insituchemical oxidative polymerization technique using ammonium per sulphate (oxidant), HCl and dodecyl benzene sulphonic acid (DBSA) as the dopant and sodium lauryl sulphate as the surfactant. The formation of polymers and blends were confirmed by FTIR, UV-visible and NMR spectroscopy. The degree of crystallinity is reduced in the blends as revealed by X-Ray diffraction studies. The polymer blends have lesser thermal stability than the polymer. The presence of DBSA reduces the thermal stability of the blend. The polymer and the blends are semiconducting and the polymer has higher conductivity than the blends. The presence of DBSA enhances the conductivity of the blend. The temperature dependent DC conductivity obeys the Arrhenius equation and the activation energy was found to be around 0.1eV for the polymer and the blends. The increase in conductivity with increase in the temperature suggested the electron hopping mechanism. The P2ClAn-DBSA/Silk has higher dielectric constant than the P2ClAn-DBSA and can find applications in the energy storage devices.
Keywords: Poly(2-chloroaniline), Polypropyleneglycol, Dodecyl benzene sulphonic acid, Electricalconductivity, Blends
Cite this paper: Porselvi Linganathan, Jhancy Mary Samuel, Effect of Dodecyl Benzene Sulphonic Acid on the Electrical Conductivity Behaviour of Poly(2-chloroaniline) and Poly(2-chloroaniline)/Silk Blends, American Journal of Polymer Science, Vol. 4 No. 4, 2014, pp. 107-116. doi: 10.5923/j.ajps.20140404.02.
Article Outline
1. Introduction
- Typical conducting polymers include polyacetylene, polyaniline, polypyrrole, polythiophene, poly (para phenylene), poly (phenylenevinylene), polyfuran etc. [1]. Among the whole conducting polymers, polyaniline has a specific situation because of its simple synthesis, environmental stability and doping with protonic acids [2, 3]. The applications of polyaniline in various important fields include active electrode [4], electromagnetic shielding materials [5], microelectronic materials and electrochromic device [5], metal anti-corrosive coating [6, 7], anti-static coating [8], rechargeable batteries [9, 10], energy storage and transfer, redox micro-template [11], indicators and sensors [12, 13]. The conducting polyanilinemicroparticles can be electrostatically accelerated to hypervelocities, indicating their suitability as mimics of solar system dusts for the calibration of impact ionization detectors for spacecraft [14]. Polyaniline also shows very high gas-separation ability with the highest ideal oxygen/nitrogen separation factor of up to 30 [15]. Inspite of various advantages, polyaniline has certain limitations when it comes to its applications as it is neither soluble nor fusible in organic solvents as well as water [16]. In order to overcome such disadvantages, attempts have been made by the use of molecular design, modification of monomer structure, use of functionalized acid dopant, formation of blends/composites and copolymerization [17, 18]. The synthesis of different homopolymers and copolymers derived from anilines with electron with drawing groups has been reported [19-22]. Colloidal particles have the potential to be finely dispersed in a polymer medium due to their small size. Therefore when colloidal particles of an intrinsically conducting polymer are used to form polymer blends, fine conductive path may generate and high conductivity levels may be realized [23]. Silk, a natural polymer is a well knownconductor but there are no reports on the study of substituted polyaniline blend with silk. An attempt has been made to synthesize the poly(2-chloroaniline) blends with the natural polymer silk and study the effect of DBSA on the rate of polymerization.In the present investigation poly(2-chloroaniline) (P2ClAn-DBSA) and poly(2-chloroaniline)/Silk in the presence/absence of DBSA (P2ClAn-DBSA/Silk & P2ClAn/Silk)were prepared by insitu chemical oxidative polymerization method using hydrochloric acid and dodecyl benzene sulphonic acid as dopants, ammonium per sulphate as an oxidant and sodium lauryl sulphate as surfactant at 0-5℃. This pathway was selected because one expects that the presence of dodecyl benzene sulphonic acid in the polymer blends enhances the solubility, stability, conductivity in organic solvents and hence the processibility as reported in our previous work with clay composites [24]. The resulting polymer and polymer blends were characterized by FT-IR, UV, H1NMR and XRD studies. The thermal stability was studied by TGA and DTA analysis and the electrical conductivity was measured by four point probe method.
2. Experimental
2.1. Preparation of Poly(2-chloroaniline)
- 2-chloroaniline, ammonium per sulphate, sodium lauryl sulphate (SDS) and dodecyl benzene sulphonic acid (DBSA) were purchased from LOBA Chemic, Qualigens and Avra Synthesis Pvt. Ltd respectively and used as received.The poly(2-chloroaniline) (P2ClAn-DBSA) was synthesized by in situ chemical oxidative polymerization method [25] using ammonium per sulphate as the oxidizing agent, HCl and dodecyl benzene sulphonic acid as the dopants and sodium lauryl sulphate as the surfactant. The monomer and oxidant were taken in 1:2 mole ratio. 2-chloroaniline was suspended in 100 ml of 1 M HCl solution and placed in the freezing mixture. The oxidant, dopant and surfactant were added drop wise to the monomer with constant stirring. The polymerization was allowed to take place for 6 hours and placed at 0℃ overnight. The green coloured product was filtered, washed and dried and obtained in high yield. Adopting the same method, the poly(2-chloroaniline) -DBSA/Silk was prepared. Silk was dissolved in 1 M HCl solution and the monomer was dispersed in the water. The monomer and silk solutions were mixed and stirred for an hour for uniform dispersion and adopting the same procedure as described above, the poly(2-chloroaniline)-DBSA/Silk was prepared. The resulting product was dark green in colour. The poly(2-chloroaniline)/Silk was also prepared by the same technique in the absence of DBSA. The resulting product was blue in colour and the yield was high. The P2ClAn/Silk was partially soluble in DMSO, DMF and partially soluble in other organic solvents like methanol, acetone, etc. The P2ClAn-DBSA and P2ClAn-DBSA/Silk were soluble in DMSO, DMF and partially soluble in other organic solvents like methanol, acetone, etc.
2.2. Experimental Methods
- The FT-IR spectra of P2ClAn-DBSA, P2ClAn-DBSA/ Silk and P2ClAn/Silk blend in KBr were recorded by Thermo Nicolet, Avatar 370 spectrophotometer from 500 cm-1 to 4000 cm-1. The UV-Visible spectra were recorded from 200-800 nm using Systronics double beam spectrophotometer 2201. XRD analysis of polymer and polymer blends were performed on a Bruker AXS D8 advance X-ray diffractometer using Cu-Kα, wavelength 1.5406Å. Thermo gravimetric analyses were carried out with a Perkin Elmer STA 6000 at the heating rate of 10℃ / min from 40℃ to 750℃ under inert gas atmosphere. The complex impedance measurements were carried out on pelleted specimens of P2ClAn-DBSA, P2ClAn-DBSA/Silk and P2ClAn/Silk using a Hewlett Packard model HP4284A precision LCR meter in the frequency range 20 HZ - 3 MHZ and in the temperature range 295-373 K. Accordingly , the synthesized solid polymers and its blend were ground into fine powders and disc shaped pellets of 10 mm diameter and the required thickness were prepared by pressing the powder samples in an IR sample press under a pelletizing pressure of 7 ton/cm2 to form circular pellets. The thickness of the pellet was measured using a screw gauge.
3. Results and Discussion
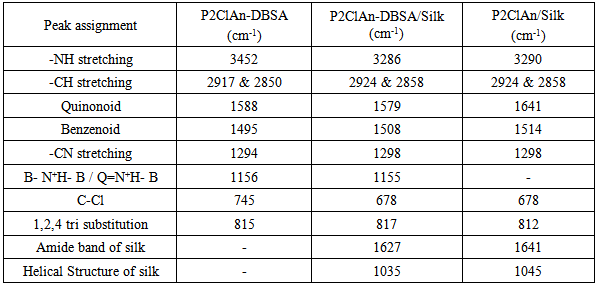
3.1. IR spectroscopy
- The IR spectra of P2ClAn-DBSA, P2ClAn-DBSA/Silk and P2ClAn/Silk blends are shown in Figure 1.1, Figure 1.2 and Figure 1.3 respectively.
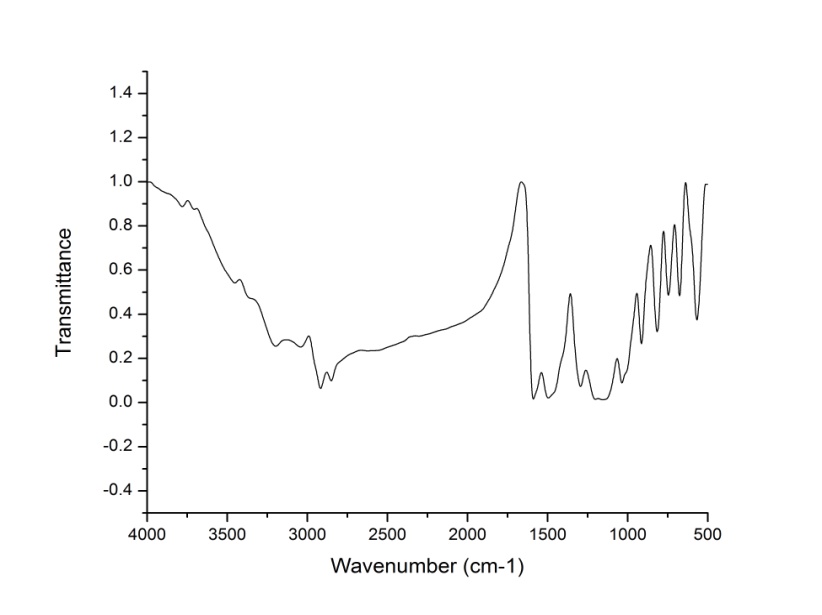 | Figure 1.1. IR spectrum of P2ClAn-DBSA |
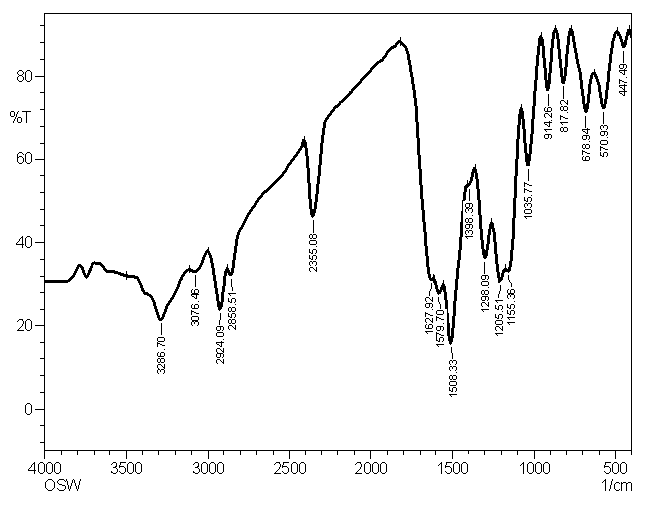 | Figure 1.2. IR spectrum of P2ClAn-DBSA/Silk |
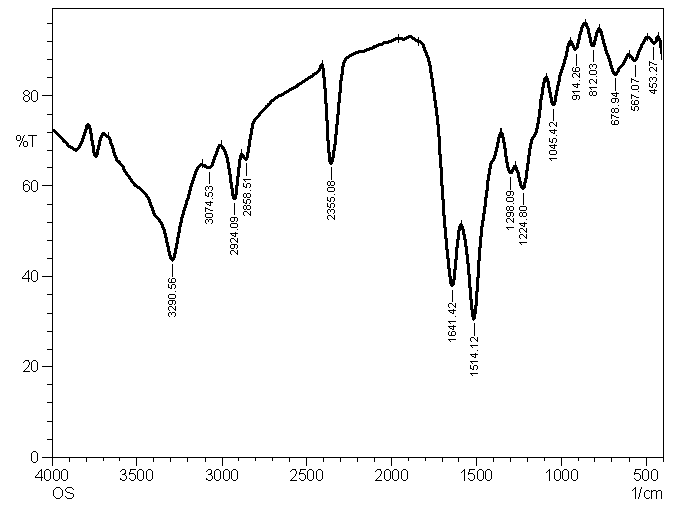 | Figure 1.3. IR spectrum of P2ClAn/Silk |
|
3.2. UV-Visible Spectroscopy
- The UV-Visible spectra of P2ClAn-DBSA, P2ClAn-DBSA/Silk and P2ClAn-DBSA/Silk are shown in Figure 2.
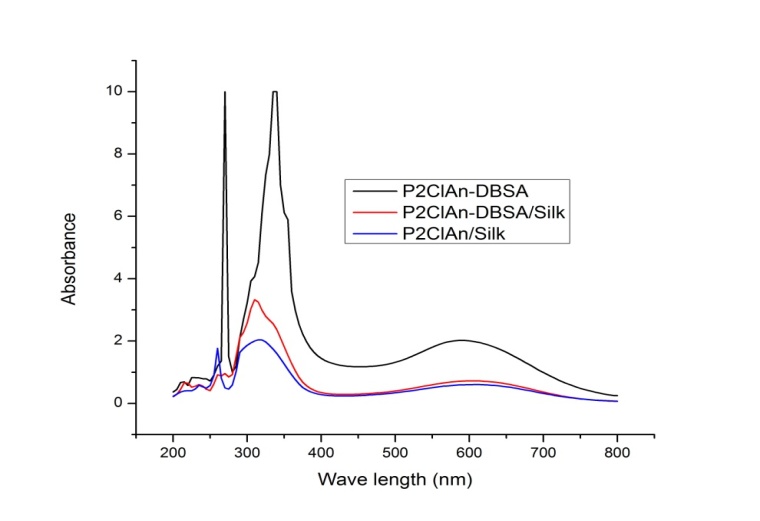 | Figure 2. UV-Visible spectra of P2ClAn-DBSA, P2ClAn-DBSA/Silk and P2ClAn/Silk |
3.3. H1 NMR Spectroscopy
- The proton NMR spectra ofP2ClAn-DBSA and P2ClAn-DBSA/Silk blends are shown in Figure 3.1 and Figure 3.2 respectively.
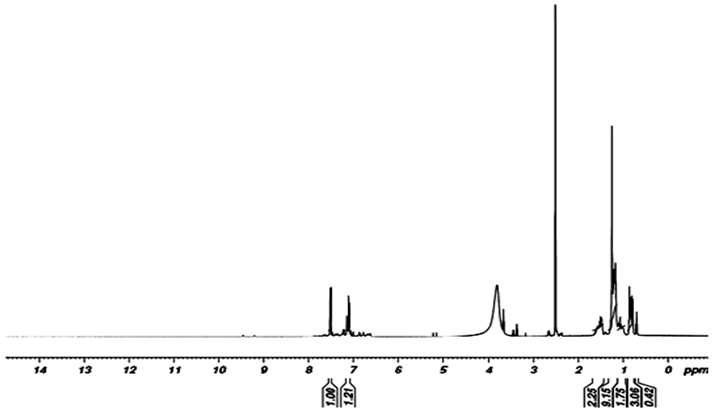 | Figure 3.1. 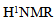 spectrum of P2ClAn-DBSA spectrum of P2ClAn-DBSA |
 | Figure 3.2.  spectrum of P2ClAn-DBSA/Silk spectrum of P2ClAn-DBSA/Silk |
3.4. X-Ray Diffraction Studies
- The XRD pattern of P2ClAn-DBSA, P2ClAn-DBSA/Silk and P2ClAn/Silk are shown in Figure 4.
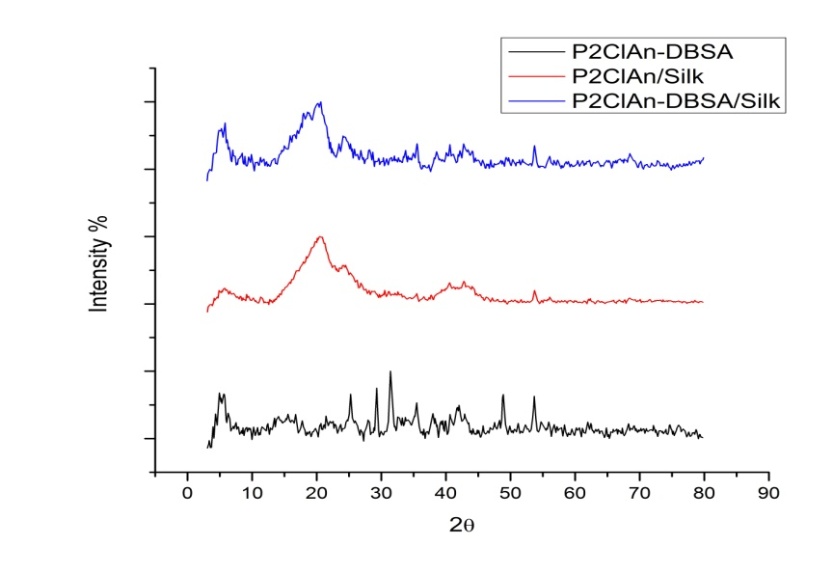 | Figure 4. XRD pattern of P2ClAn-DBSA, P2ClAn-DBSa/Silk and P2ClAn/Silk |
3.5. Thermo Gravimetric Analysis
- The TGA of P2ClAn-DBSA, P2ClAn-DBSA/Silk and P2ClAn/Silk are shown in Figure 5.
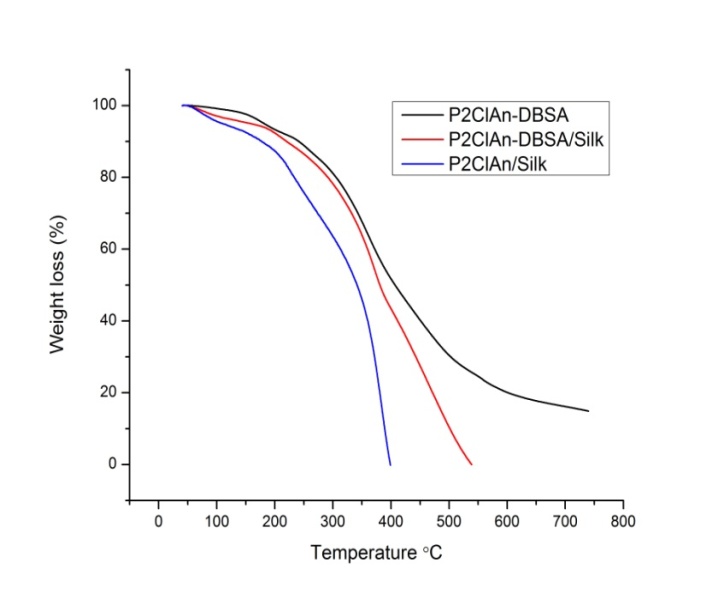 | Figure 5. TGA of P2ClAn-DBSA, P2ClAn-DBSA/Silk and P2ClAn/Silk |
3.6. Impedance Analysis
- The complex impedance plots for P2ClAn-DBSA, P2ClAn-DBSA/Silk and P2ClAn/Silk measured at various temperatures ranging from room temperature to 383K in the frequency region 50 Hz to 35 MHz are shown in Figure 6.1, Figure 6.2 and Figure 6.3 respectively.
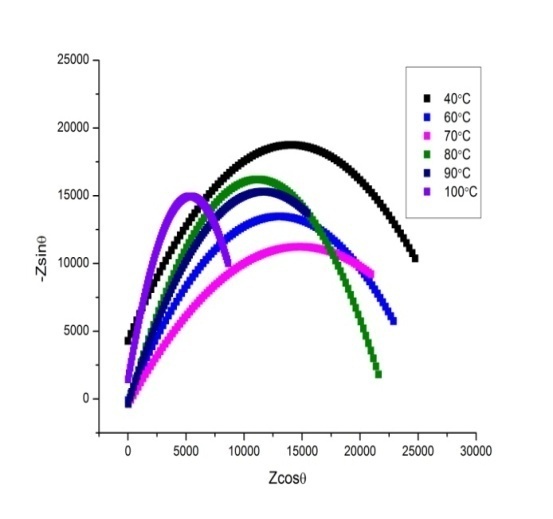 | Figure 6.1. Complex impedance plot of P2ClAn-DBSA at various temperatures |
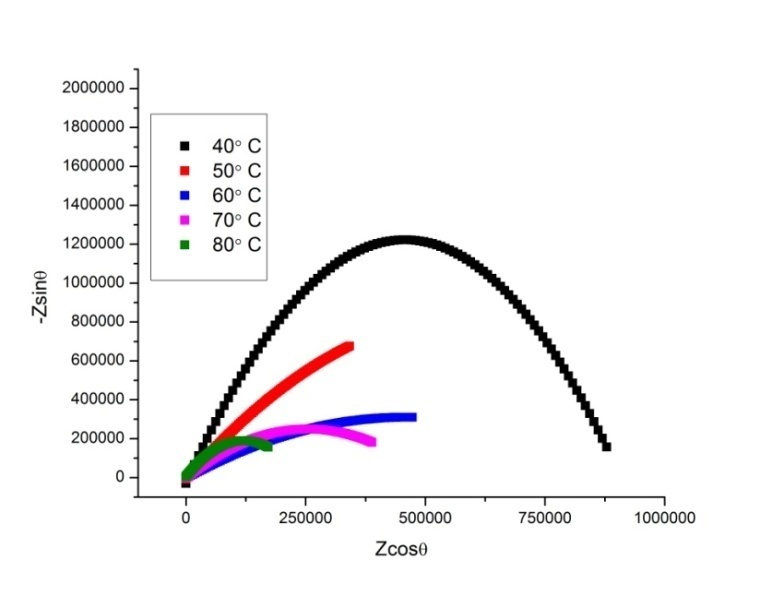 | Figure 6.2. Complex impedance plot of P2ClAn-DBSA/Silk at various temperatures |
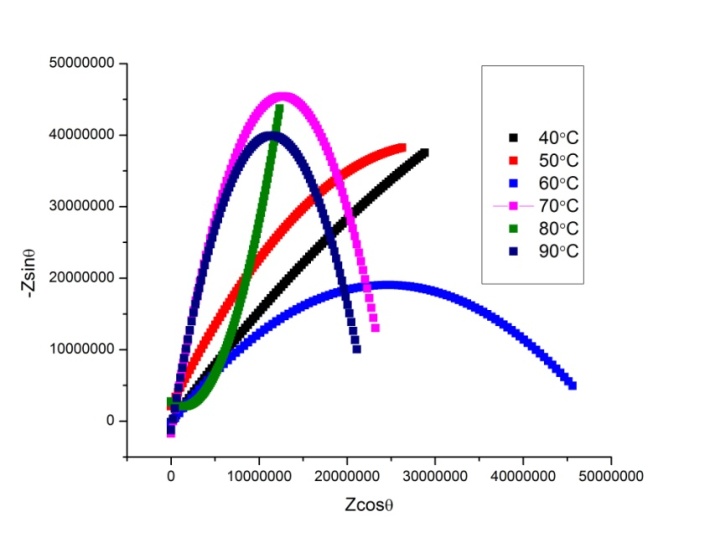 | Figure 6.3. Complex impedance plot of P2ClAn/Silk at various temperatures |
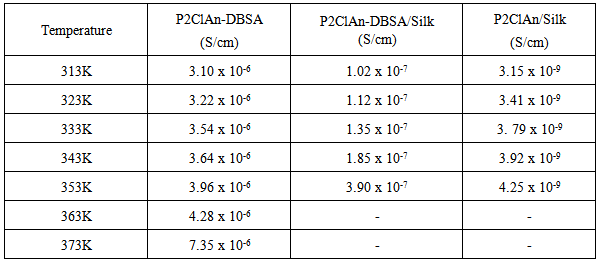
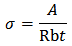 where ‘A’ is the area of the pellet and ‘t’ is the thickness of the pellet. It can be seen from the figure that as the temperature is increased, the point of intersection on the x-axis in the Cole-Cole plot is shifted towards the origin. Hence, it is evident that the bulk resistance of the polymer decreases with the increase in temperature, resulting in the enhancement of electrical conductivity at higher temperatures. The temperature dependent conductivity values for P2ClAn-DBSA, P2ClAn-DBSA/Silk and P2ClAn/Silk reveal that the conductivity increases with temperature. The conductivity value of P2ClAn-DBSA, P2ClAn-DBSA/Silk and P2ClAn/Silkat various temperatures are tabulated in Table 2.
where ‘A’ is the area of the pellet and ‘t’ is the thickness of the pellet. It can be seen from the figure that as the temperature is increased, the point of intersection on the x-axis in the Cole-Cole plot is shifted towards the origin. Hence, it is evident that the bulk resistance of the polymer decreases with the increase in temperature, resulting in the enhancement of electrical conductivity at higher temperatures. The temperature dependent conductivity values for P2ClAn-DBSA, P2ClAn-DBSA/Silk and P2ClAn/Silk reveal that the conductivity increases with temperature. The conductivity value of P2ClAn-DBSA, P2ClAn-DBSA/Silk and P2ClAn/Silkat various temperatures are tabulated in Table 2.
|
3.7. Temperature Dependent Conductivity
- The variation of conductivity as the function of inverse temperature for the P2ClAn-DBSA, P2ClAn-DBSA/Silk and P2ClAn/Silk are in the Figure 7. In the form of plots of
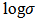 versus
versus 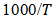 where
where 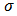 denotes the conductivity and 𝑇 the absolute temperature.
denotes the conductivity and 𝑇 the absolute temperature.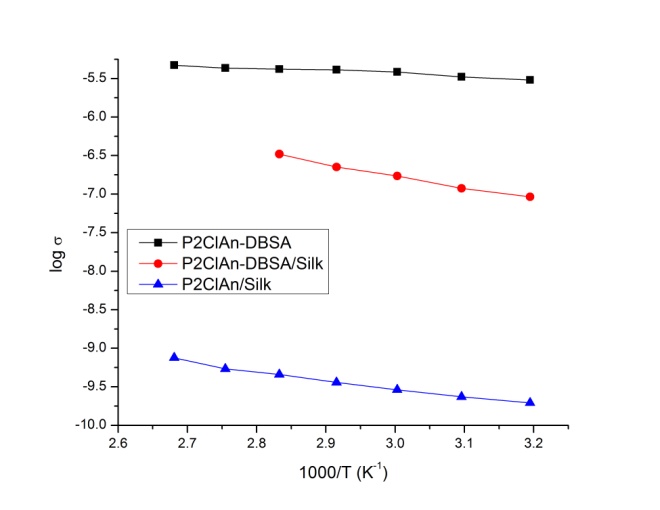 | Figure 7. Arrhenius plot of P2ClAn-DBSA, P2ClAn-DBSA/Silk and P2ClAn/Silk |
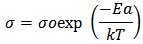 where
where 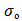 is the pre-exponential factor,
is the pre-exponential factor, 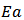 is the activation energy and k is the Boltzmann constant. The increase in conductivity with increase in temperature suggested the electron hopping mechanism. As the temperature increases the polymer chain acquires faster internal modes in which bond rotations produce segmental motion. This in turn favors hopping inter-chain and intra-chain ion movements and accordingly the conductivity of the polymer becomes high [31]. The activation energy was calculated from the slope of the observed linear plot drawn by the least square method and was found to be around 0.1 eV for the polymer and the blends.
is the activation energy and k is the Boltzmann constant. The increase in conductivity with increase in temperature suggested the electron hopping mechanism. As the temperature increases the polymer chain acquires faster internal modes in which bond rotations produce segmental motion. This in turn favors hopping inter-chain and intra-chain ion movements and accordingly the conductivity of the polymer becomes high [31]. The activation energy was calculated from the slope of the observed linear plot drawn by the least square method and was found to be around 0.1 eV for the polymer and the blends.3.8. Conductance Spectra
- The conductance spectra show the relation between the conductivity and the frequency. The frequency dependent AC conductivity of P2ClAn-DBSA, P2ClAn-DBSA/Silk and P2ClAn/Silk at various temperatures are shown in Figure 8.1, Figure 8.2 and Figure 8.3.
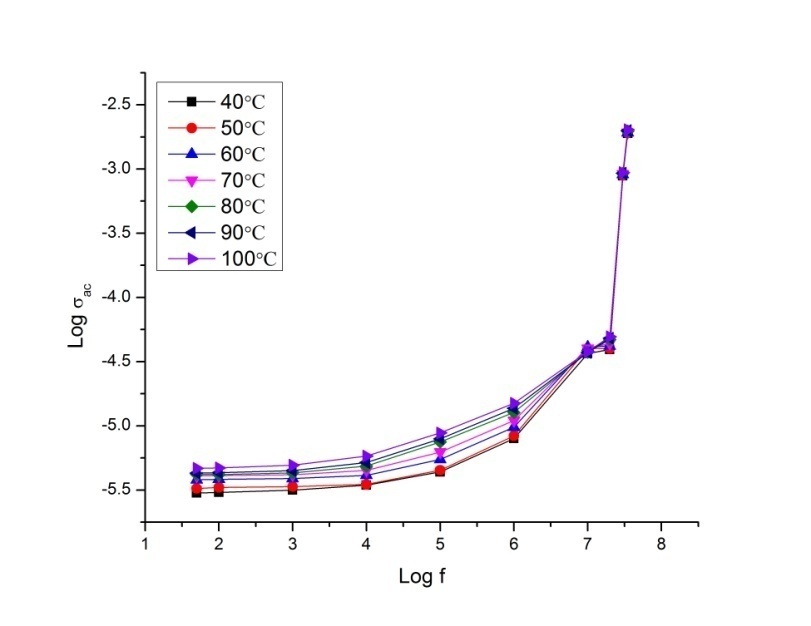 | Figure 8.1. Conductance spectrum of P2ClAn-DBSA at various temperatures |
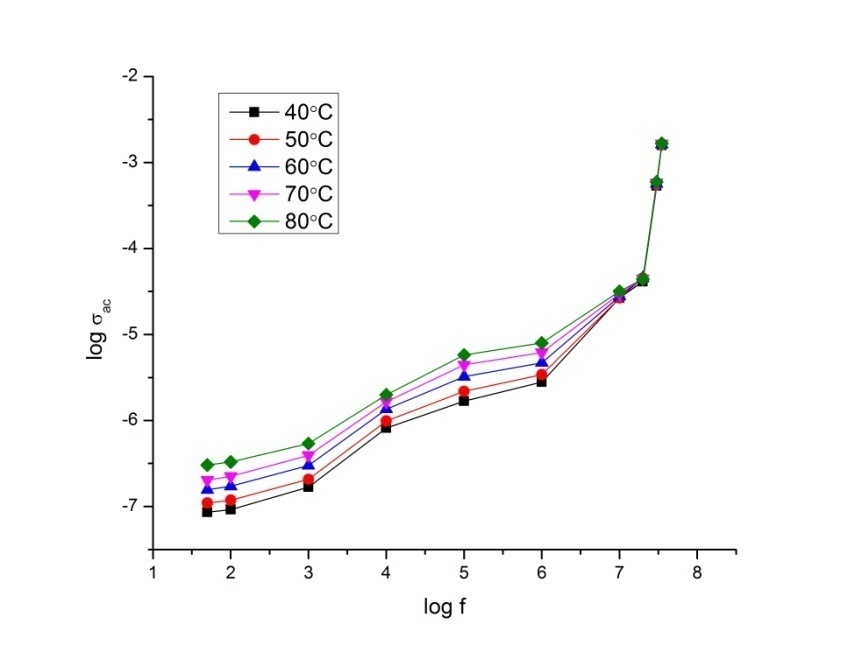 | Figure 8.2. Conductance spectrum of P2ClAn-DBSA/Silk at various temperatures |
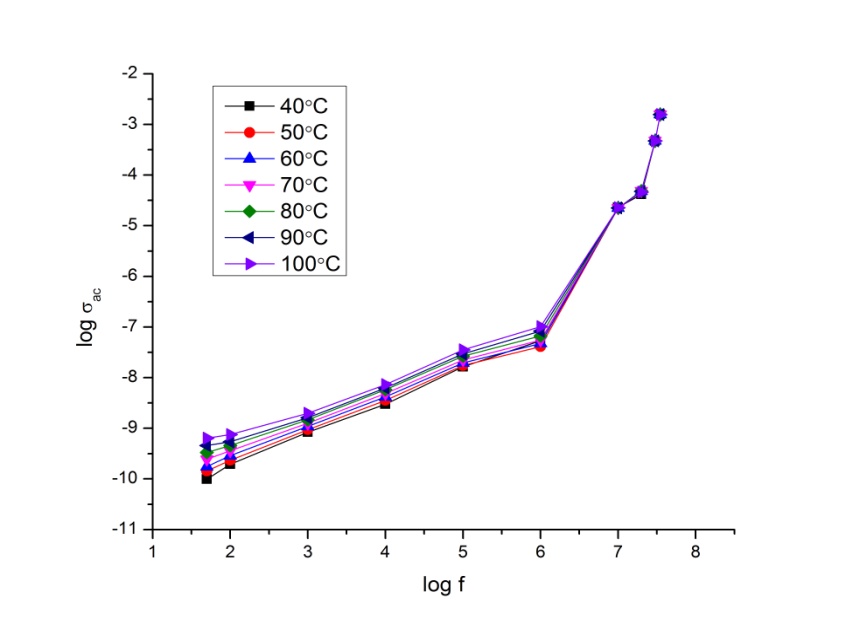 | Figure 8.3. Conductance spectrum of P2ClAn/Silk at various temperatures |
3.9. Dielectric Properties
- The dielectric properties of conducting polymers are one of the main focus points of research because of their novel technological applications. It is well established that the polymers, as dielectric materials, are excellent host matrices and also provide environmental and chemical stability [33]. The dielectric constant
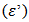 has been calculated from the measured values of capacitance using the following formula
has been calculated from the measured values of capacitance using the following formula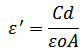 where
where 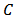 is the measured capacitance of the sample,
is the measured capacitance of the sample, 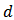 is the thickness of the sample,
is the thickness of the sample, 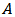 is the area of the pellet, and
is the area of the pellet, and 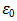 is the permittivity of free space.Figure 9.1, Figure 9.2 and Figure 9.3 indicate the variation of dielectric constant of P2ClAn-DBSA, P2Clan-DBSA/Silk and P2ClAn/Silk with respect to the frequency with various temperature ranges.
is the permittivity of free space.Figure 9.1, Figure 9.2 and Figure 9.3 indicate the variation of dielectric constant of P2ClAn-DBSA, P2Clan-DBSA/Silk and P2ClAn/Silk with respect to the frequency with various temperature ranges.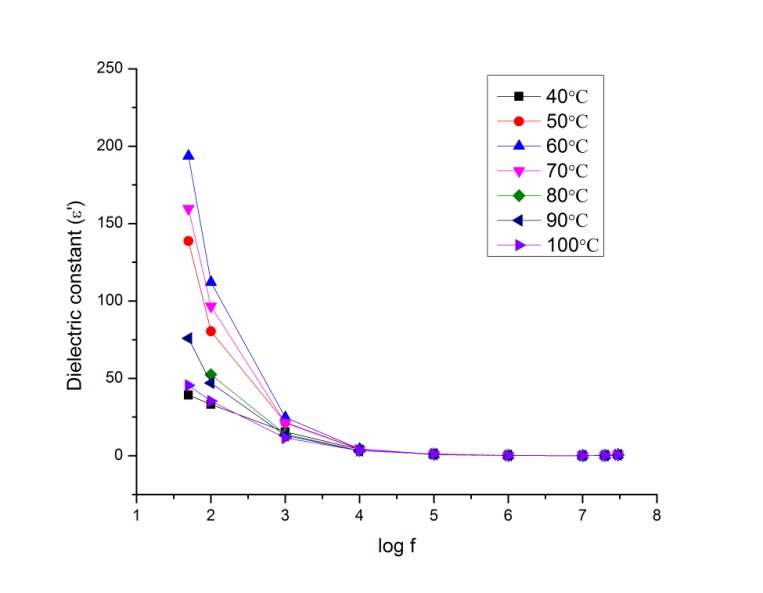 | Figure 9.1. Variation of dielectric constant as a function of frequency for P2ClAn-DBSA at different temperatures |
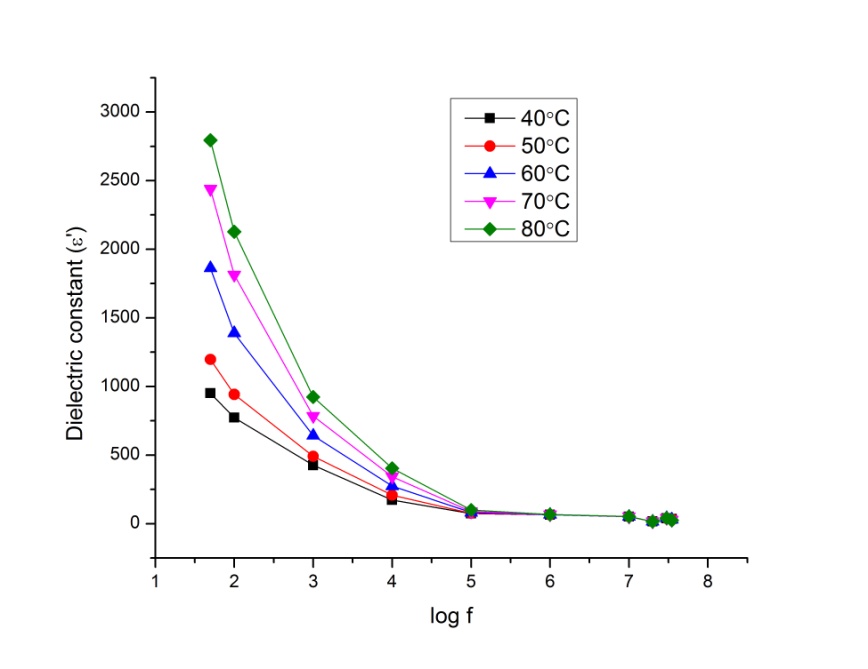 | Figure 9.2. Variation of dielectric constant as a function of frequency for P2ClAn-DBSA/Silk at different temperatures |
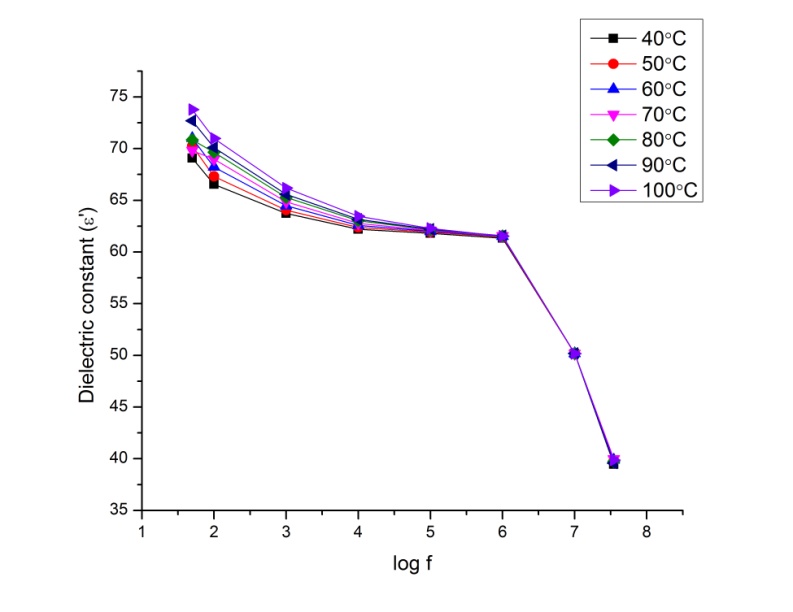 | Figure 9.3. Variation of dielectric constant as a function of frequency for P2ClAn/Silk at different temperatures |
3.10. Proposed Structure of the Polymer Blend
- The formation of blend between the polymer and silk was confirmed by the spectroscopic techniques. Silk in its raw state consists of two main proteins, sericin and fibroin. The maximum part of the silk contains the amide group which is present in the aminoacids. The interaction may be due to the hydrogen bonding between the hydrogen present in the polymer and the oxygen present in the amide group of the silk. Figure 10 designates the schematic representation of the proposed structure.
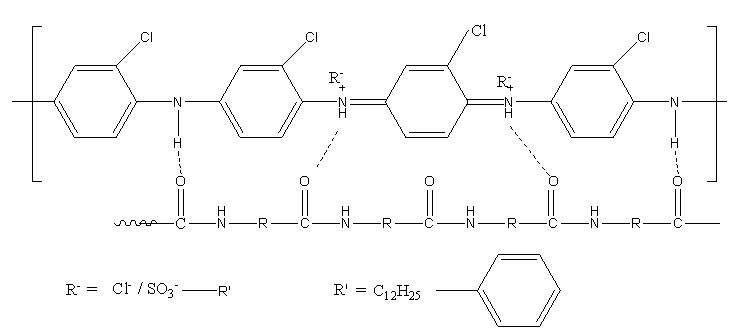 | Figure 10. Schematic representation of the proposed structure |
4. Conclusions
- The P2ClAn-DBSA, P2ClAn-DBSA/Silk and P2ClAn/Silk were successfully synthesized by insitu chemical oxidative polymerization method. The synthesized polymer and its blends were characterized using IR, UV, H1NMR and XRD. The presence of benzenoid and quinonoid rings indicates the formation of polymer and the blends. The ratio of the area of benzenoid and the quinonoid rings suggest the emeraldine salt structure. The crystallinity of the polymer is reduced by the formation of blends. The P2ClAn-DBSA shows higher conductivity than the polymer. While comparing the conductivity of the silk blends, the enhanced conductivity is observed in the presence of DBSA due to formation of emeraldine salt structure. Whereas in the absence of DBSA, the emeraldine base structure is formed and hence a low conductivity is observed. The temperature dependent DC conductivity has been obeying the Arrhenius equation. The activation energy of the polymer and blends are found to be 0.1 eV. The P2ClAn-DBSA/Silk has higher dielectric constant than P2ClAn-DBSA and P2ClAn/Silk, hence it can be used in the energy storage devices.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML