-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Polymer Science
p-ISSN: 2163-1344 e-ISSN: 2163-1352
2014; 4(4): 101-106
doi:10.5923/j.ajps.20140404.01
Modification of Adhesive Using Cellulose Micro-fiber (CMF) from Melon Seed Shell
Aibudefe Pius, Lawrence Ekebafe, Stella Ugbesia, Rosabel Pius
Polymer Technology Department, Auchi Polytechnic, Auchi, Nigeria
Correspondence to: Aibudefe Pius, Polymer Technology Department, Auchi Polytechnic, Auchi, Nigeria.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Melon seed shell was de-shelled, broken down into smaller pieces and then hydrolyzed with 10%(w/w) of nitric acid; oven-dried at 960c for 30mins and then bleached with sodium hypochlorite with 10% chlorine for 24hrs and washed with distilled water and dried. The dried chemically modified melon peel (CMF) were ground using a local mill and sieved to get a fine dispersion of dried melon peel filler and a higher dispersion in the adhesive. The powdered melon seed shell and CMF are chemically analyzed using standard methods. The adhesive was prepared using poly (vinyl alcohol) as reinforcing additive; it was characterized and modified using cellulose microfiber from melon seed shell. The result of the CMF modified fiber adhesive in terms of bond strength, and peel strength revealed that melon seed shell can favorably be used as filler in adhesive manufacturing and also reduce environment waste. The result of the parameters studied before and after treatment of the adhesive show that cellulose microfiber melon seed shell is effective as filler in adhesive production.
Keywords: Adhesive, Cellulose microfiber and melon seed shell
Cite this paper: Aibudefe Pius, Lawrence Ekebafe, Stella Ugbesia, Rosabel Pius, Modification of Adhesive Using Cellulose Micro-fiber (CMF) from Melon Seed Shell, American Journal of Polymer Science, Vol. 4 No. 4, 2014, pp. 101-106. doi: 10.5923/j.ajps.20140404.01.
Article Outline
1. Introduction
- Melon seeds are most useful in most parts of southern Nigeria and the seed shell is a major issue of environmental pollution in these parts of the country.Cellulose microfibers (CMF) are can be extracted from some plants or plant – based materials in this study from melon seed shell [1, 2]. The reason for using CMF as filler in adhesive in this study is to improve on cohesive bond properties of the adhesive itself. Melon seed shell is a readily available waste material: this also indicates that this method is a means of eradicating or reducing environmental waste from melon seed.This research uses the flexible qualitative research method. The initial stage of the adhesive was actively changed which leads to a modification in behavior or performance of the adhesive by the incorporation of CMF into the matrix of the adhesive. Low cost, availability and simple methods for synthesis and application have been the main parameters considered when selecting materials to be applied in this area [3]. Special effort has been devoted to finding alternative sources of cellulose microfibers as fillers, given its effectiveness and simple techniques for it production.Cellulose microfibers as filler are becoming more and more applicable in the polymer industries, where it is used as fillers in biodegradable products without without reacting with the polymer. The use of CMF as reinforcement can lead to composites with high specific properties. Cellulose fibers flexibility enables the fibers to maintain the desired aspect ratio for good performance. Their non-abrasive nature permits a high volume of filling during processing which results in high mechanical properties [4-6].Several workers have reported on the potential use of agricultural by-products as good substrates for reinforcement such as cocoa pod husks [7], rubber seed shall [8], fly –ash [9], Maize starch [10], Oil palm Micro fibril [11], saw dusts [12], Wheat and rice husk [13] etc.This process attempts to put into use the principle of using waste as reinforcement and become even more efficient because these agricultural by products are readily available and often pose waste disposal problems. As waste products they are available at little or no cost. This makes polymer reinforcement with agricultural by products more cost effective. Melon seed (Cucumeropsis mannii) is a species of melon native to tropical Africa west of the east African rift, where it is grown for food. Adhesive or stick-on is a material usually in a liquid or semi-liquid stage that adheres or bond items together. An adhesive can come from either natural or synthetic sources. The types of materials that can be bonded are vast but adhesive are especially useful for bonding thin materials. Adhesive cure (harden) by either evaporating the solvent or chemical reactions that occur between two or more constituents. Adhesives are advantageous for joining thin or dissimilar materials, minimizing weight and providing a vibration damping joint; there is the polymer dispersion adhesive (PDA) which are milky white dispersion based on polyvinyl acetate (PVA) [14-17]. They are used extensively in the wood working and packaging industries-also used with fabric and fabric based component, and in engineered products such as loud speaker cones; there is also the pressure Sensitive Adhesive (PSA):- these form a bond by the application of light pressure to marry the adhesive with the adhered. They are designed with a balance between flow and resistance to flow. The bond form because the adhesive if soft enough to flow (wet) to the adhered. The bond has the strength because the bond is strong enough to resist flow when stress is applied to the bond. Once the adhesive and adhered are in close proximity. Molecular interactions, such as van der waals forces become involved in the bond, contributing significantly to its ultimate strength [15-16]. PSAS are designed for either permanent or removable applications. Examples of permanent applications include safety labels for power equipment automotive interiors trim assembly etc [17-20]. This present study is aimed at investigating the potentials of CMF from melon seed shell as reinforcement (filler) for adhesive.
2. Materials and Methods
- Melon seed shell was sourced from a local melon seed mill in Auchi, Edo state, Nigeria. Polyvinyl alcohol and distilled water used for the preparation of the adhesive, nitric acid and sodium hypochlorite used for the pretreatment of the melon seed shell were products of Sigma-Aldrich, Germany.
2.1. Isolation of the Cellulose Microfibre
- The melon seed shell was cut into smaller pieces and then hydrolyzed with 10%(w/w) of nitric acid and heat for 96% for 30 mins the pulp was bleached with sodium hypochlorite the CMF was isolated from the oven dried melon seed shell with 10% chlorine. The bleaching was done by measuring 10 ml of sodium hypochlorite and dissolving it in 2 liters of distilled water in a container, the water foams due to excess acidic content and allowed to dissolve by stirring to get a clean solution. The pulp was poured into the mixture and left for 24 hrs to allow proper soaking and bleaching, after which it was filtered, washed with distilled water and dried [21]. The CMF isolated from the oven – dried melon seed shell were chemically analyzed for moisture and ask contents by using the A.D.A.C (1999) standard. The isolation was studied with FTIR.
2.2. Characterizations of the Isolated Cellulose Microfibers
- Chemical Analysis of melon fibres and cellulose microfibers (CMF)The melon fibres and CMF were chemically analyzed for moisture and ash contents by using the A.O.A.C (1999) standard. The cellulose content of the fibres was measured by using Kurschner and Hoffer method [22]. It was also characterized in terms of the lignin content, Holocellulose content, and Hemicellulose content according to standard methods.
2.3. Modification of the Adhesive
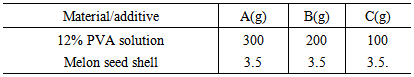
- The dried chemically modified melon peel were ground using a local mill and sieved in a mesh size of 75 µm in diameter; to get a fine dispersion of dried melon peel filler and to get high filler dispersion in the adhesive. 20g of melon seed shell filler was used in the preparation of the CMF filler slurry (paste) by dissolving it in 150ml of distilled water. The production of the adhesive was carried out by adding the additive according to the formulation in the table below for samples a, b and c, which was stored in a clean labeled plastic container.Bond strength test was carried out to determine the strength of the adhesive; this is done by applying the adhesive sample each to paper-paper, paper-glass and paper-wood (which can be referred to as test piece) and allowed to dry for 6 hrs. The test piece was subjected to various loads for 1hr: 30mins to determine their bond strength. Peel strength test was also carried out to determine the peel strength of the adhesive the test was done by applying small force on the already prepared test piece(s) in separating them and the rate at which they were separated for 30 mins was recorded [23].
|
2.4. Testing of Adhesive
- The adhesives were subjected to analysis to determine their properties. These included the setting speed of the adhesives and the mechanical properties of PVOH adhesives were measured as the shear strength and elongation-at-break (EAB) according to TIS.360-2523 and ASTM D1002-72, 1982. The specimens for mechanical testing were prepared from a 0.5 cm thick plane wood which was cut to a dimension of 2.5×10 cm2. Small quantities (0.05g) of the adhesives were applied to the surface of the wood in contact with another piece of plane wood. 5 test specimens were used. The glued plain wood were then pressed together under pressure at ambient temperature. Test samples were maintained and conditioned at 23°C for 2 days before testing.
3. Result and Discussion
3.1. Effect of Chemical Treatment on Fibre Characterizations
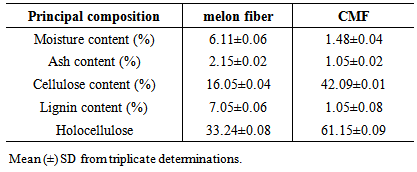
- Chemical Compositions of the melon fibre and CMFThe melon fibres and CMF were chemically analyzed to measure the moisture, ash, cellulose and cellulose contents. Before testing, the fibres were prepared by grinding and screening with a sieve, mesh No. 75/60. Table 2 shows some of the chemical compositions of the fibres. The moisture and ash contents of melon fibres are 6.11% and 1.48%, respectively. The CMF showed moisture and ash contents of 2.15% and 1.05%, respectively.
|
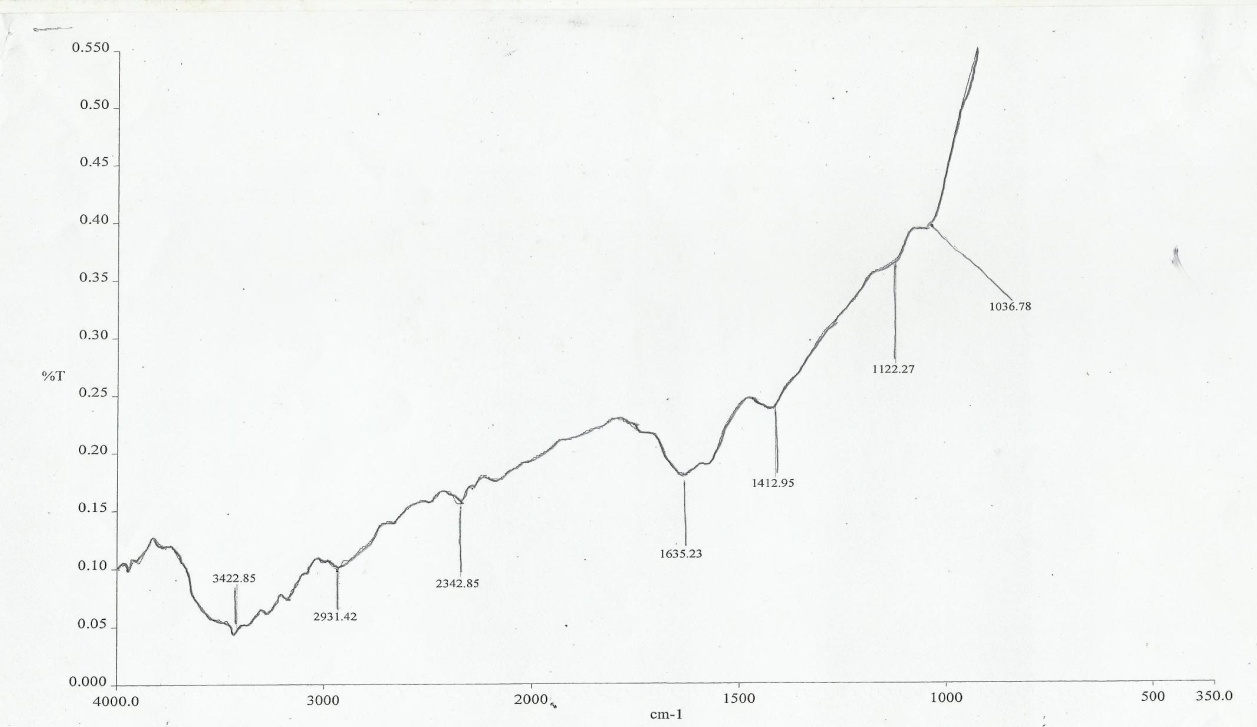 | Figure 1. FTIR Spectra of the melon seed shell |
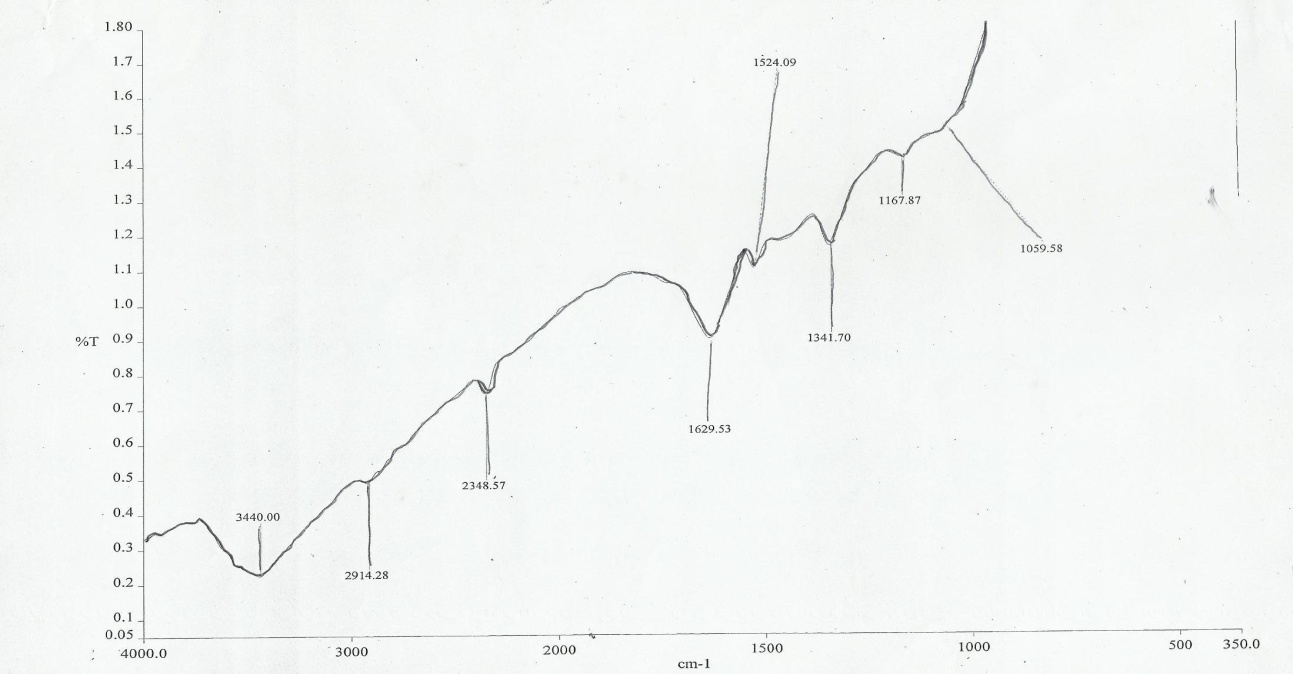 | Figure 2. FTIR Spectra of the CMF |
3.2. Fourier Transform IR Spectroscopy (FTIR) Characterization of Melon Seed Shell Fibers and CME
- The chemical structures of the melon seed shell fibers and CMF component show strong characteristic carbonyl absorption peak at 1635.23cm-1. This was attributed to the acetyl and Uronic ester group of the hemicelluloses, the carboxylate groups of the ferulic and p-coumeric acids of lignin hemicelluse, [28, 29]. The peaks at 163.33 and 1412.95cm-1 in the melon fibers show the aromatic c=c stretch of the aromatic rings of lignin [28, 30]. The FTIR spectra of CMF shows the removal of pectin’s, lignin and hemicelluloses resulting from the vanishing characteristic band at 1629.53cm-1 (carboxylate groups), 1341.789cm-1 (methyl ester groups), 1629.53 and 1524. 09cm-1 (aromatic C=C stretch), indicating that the treatment had removed large amounts of pectin, lignin and hemicelluloses. Equally this was in accordance with the chemical changes in the surface and its crystallinity in literature [29].
3.3. Effect of CMF Addition on PVOH Composite Adhesives Properties
3.3.1. Shear Properties of the PVOH Composite Adhesives
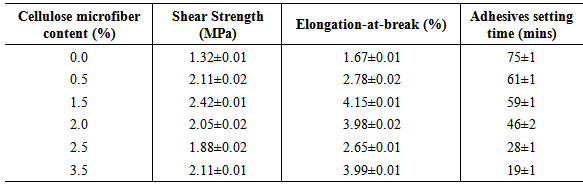
- Shear strength and elongation-at-break are the principal parameters relating to the mechanical properties of adhesives. The shear strength of adhesives also affects failures in veneers. If there is higher shear strength, there is also higher veneer failure strength [31]. The improvement of the mechanical properties of PVOH adhesives by CMF were investigated in this study. The criteria for judging PVOH adhesives for mechanical properties improvement were shear strength, elongation at break and compatibility with additives.Table 3 shows the shear strength of PVOH adhesives containing different quantities of CMF (0-3.5 wt %). The shear strength of the PVOH adhesives increased from 1.32 to 2.42 MPa when increasing the CMF filler content from 0 to 1.5 wt%. The results showed that the maximum shear force that was capable of separating or de-bonding joined hard-wood pieces glued together was an indication of strong interactions between the CMF and PVOH adhesive through hydrogen bonding. Similar results have been reported in PVOH/CMF composite films by Bhatnagar [32]. However, the addition of CMF at 2.0 to 3.5 wt% could induce an accumulation which actually decreases the effective content of the CMF. Thus, PVOH adhesives with 2.0 to 3.5 wt% CMF contents exhibited lower shear strength compared to samples reinforced with 0.5 and 1.5 wt% CMF.
|
3.3.2. Adhesive Setting Time
- The quick setting time of adhesives is another requirement for the application of wood adhesives.The PVOH adhesives consist of PVOH dissolved in water. Thus, the water is used as a solvent. The adhesives are "set" when a certain amount of water is evaporated [34]. Table 3 shows the effect of the amount of cellulose micro fibre on the setting speed of PVOH adhesives. The setting time of PVOH adhesives has been accelerated with increased amount of CMF. The setting time of PVOH adhesives containing CMF contents of 0- 3.5 wt% are 19 -75 mins.It may be concluded that the CMF is able to improve the setting speed of PVOH adhesives.Since cellulose is a hydrophilic polymer and a water-absorbing material, there are strong interactions between hydroxyl groups at the cellulose surface, PVOH polymer and water or solvent [33]. The adhesion of glues has been thus improved. However, the other potential effects of setting speed still depend upon the type of solvents, air-blowing steps and the evaporation temperature [35].
4. Conclusions
- CMF extracted from melon seed shell and the result show that the CMF is effective as reinforcement in adhesive technology. It improves the physical properties of the adhesive like bond strength and peel strength, 2.42 and 2.92 MPa at 1.5% respectively which when compared with the unfilled adhesive proves to be better. CMF from melon seed shell is readily available and can degrade without reacting with the polymer.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML