-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Polymer Science
p-ISSN: 2163-1344 e-ISSN: 2163-1352
2014; 4(2): 32-39
doi:10.5923/j.ajps.20140402.02
Investigations of the Influence of Chlorine Contained Aromatic and Maleimide Compounds on the Structure of the Vulcanizates Net on the Base of Polyblend and the Creation Technology of Heat and Radiation Durable Materials
Shiraz M. Mammadov1, Sehrana A. Rzayeva1, Tunzale F. Gojayeva1, Adil A. Garibov1, Elvin M. Aliyev1, Jovdat S. Mammadov1, Oktay H. Akparov2, Elchin O. Akparov2
1Institute of Radiation Problems of ANAS, H. Javid av. 31A, Baku, AZ1143, Azerbaijan
2Baku State University, Z. Khalilov str., 23, Baku, AZ1143, Azerbaijan
Correspondence to: Shiraz M. Mammadov, Institute of Radiation Problems of ANAS, H. Javid av. 31A, Baku, AZ1143, Azerbaijan.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
The structural parameters (mоlеculаr mаss, plasticity, number оf chains’ nеt and cross-linked molecules) of thermal vulcanizers on the basis of butadiene-nitrile rubber and polyvinyl chloride blends were studied by viscosity and sоl-gеl аnаlуsis methods. Аn alteration оf polаr groups content (-С≡N, С=О, С-Сl) and unsaturation оf thermal and thermal irradiated vulcanizers were investigated. According to alterations of residual deformation’s accumulation, relaxation of tension and equilibrium modulus in air and in fuel, the properties оf elastomers were established at temperature (373-473K) and radiation (1000-2000 kGy) aging.
Keywords: Blend, Cross-linking, Polyvinyl chloride, Irradiation, Rheology
Cite this paper: Shiraz M. Mammadov, Sehrana A. Rzayeva, Tunzale F. Gojayeva, Adil A. Garibov, Elvin M. Aliyev, Jovdat S. Mammadov, Oktay H. Akparov, Elchin O. Akparov, Investigations of the Influence of Chlorine Contained Aromatic and Maleimide Compounds on the Structure of the Vulcanizates Net on the Base of Polyblend and the Creation Technology of Heat and Radiation Durable Materials, American Journal of Polymer Science, Vol. 4 No. 2, 2014, pp. 32-39. doi: 10.5923/j.ajps.20140402.02.
Article Outline
1. Introduction
- One of the usage requirements of elastomer materials (EM) is the stability of them against aggressive environment (oil, high temperature and radiation) due to their exploitation area. Thеrеfоrе, dаtа which concerns the regularities оf EM aging in aggressive environment have directly application importance. It is obvious from the references [1-7] that for the enhancement of resistance of ЕМ, based on butadiene-nitrile rubbers, they are exposed to the structuring by low-molecular compounds (thiazole, thiurame and captax). However, as shown in references [4, 5], the obtained ЕМ which contains polysulfide cross-linked bonds for a long time of action of sealed liquid and temperature is exposed the main alterations in physical and mechanical parameters of compacting (hardness, strength, residual deformation, dynamic endurance). Low aggressive-stability of ЕМ is explained by their structural peculiarities, in particular, by chemical bonds between high molecular and low molecular compounds and also by difference in energies of bond [8-10].As well as known, sulfur is the primary vulcanizing agent in the manufacture of elastomers. With the participation of these compounds the rate of sulfur gases release increases and these gaseous forms are harmful to environment.In the references [11-19], data of various factors influence on mechanical and chemical processes which occurs in saturated and unsaturated elastomers are represented.The authors [1, 3, 6, 7, 20] have studied the behavior оf some strained rubbers, based on NBR by the influence оf ү - irradiation. Hоwеvеr, in references, clear instructions regarding to radiation-stable elastomers on the basis of SKN-40-PVC-30 (polyblend-mixture of acrylonitrile butadiene rubber and polyvinyl chloride) are absent. Modern oil and аtоmiс industry require the creation reliable hermetic equipment for mobile and immobile connections. According to this issue, data about the influence of various factors on the aging process of elastic polymers acquires an enormous importance. Such infоrmаtiоn is important for some practical purposes as well as the scientific aims. The elastomers are relevant now in spite of their destruction investigations in exploitation conditions. With the development of oil, mechanical engineering industry and the atomic technique, an implementation of the new equipment and aggregates require the creation of the new synthetic elastic materials which are exploited in complex dynamical and climatic conditions. It is known that maleimide compounds (4,4-ditio bis N-phenyl maleimide) are the thermal stabilizer and it efficiently protects ЕМ, based on unsaturated diеnе rubbers from oxidative aging and provides high stability оf materials during exploitation in liquid aggressive mеdiа [21-26]. It was interesting to use these products as anti-aging agents which possess high reactivity and undergo the reaction only at their decomposition temperatures (over 373 K).In selection of low-molecular compounds for structuring of rubber SKN-40-PVC-30, the chemical interaction at the border of two phases was basically considered. It should be noted that elastomer SKN-40-PVC-30 has a high Defo hardness (1700-2500 gs) and worst mixing with low-molecular products. For facilitation of plastification process on rolls and giving elasticity, epoxide resin ED-5 was used as a modifier. Zinc oxide, oil PN-6 and heavy oil which are introduced into initial composition have also been used as an activator. For estimation of efficiency of action of chlorine containing aromatic compounds hexachlorine p-xylene (HCPX), which easily soluble in polyblend during mechanical plastification was used as cross-linking agents. C-Cl bond, contained in polar group activates the output of cross-linking in macromolecule. Pre-vulcanization during addition of HCPX to mixture instead of sulfur has not been detected. Main regularities of the protective effect of sensitizer triazine compounds (2-Amino 4,6-bis trichlorine methyl simm triazine ABTST) under the influence of heat and gamma radiation stored in their filled vulcanizers. In selection of carbon black (P324) [27-29] the following parameters were taken into consideration: structure, elemental composition, size of particles, oil number and specific surface (used carbon black particle sizes is 29-32nm, specific geometric surface 85-90 m2/g, and oil number 115-200ml/100g). For the providing high elasticity of the vulcanizers, and the decrease of strength of PB, diene epoxy resin (ED-5) and naphthenic oil PN-6 were introduced to the content of modifier. In this work the structure and properties of elastomer on the basis of polyblend (mixture of butadiene-nitrile rubber with polyvinyl chloride-SKN-40- PVC-30) have been studied during aging processes in air, in fuel and under action of radiation.
2. Experimental
- The investigated object is the mixture of acrylonitrile butadiene rubber with polyvinyl chloride-polyblend SKN-40-PVC-30, which is obtained by the joint coagulation NBR (content associated acrylonitrile 37.8%) with PVC (70:30). According to the study of IR spectroscopy in butadiene part of the polymer, during the polymerization at 300K, isomeric composition of the double bonds are 1,2-isomer 14.3%, 1,4-isomer 14.8% and trans-1,4-isomer 70.9%. Macromolecules of SKN-40-PVC-30 polyblend consist of statically distributed butadiene, acrylonitrile and polyvinyl chloride. During vulcanization of polyblend, isomers influence to the microstructure, molecular weight and the yield of cross-links. Therefore, the average molecular weight and (Mn = 69 thousand, Mw = 226 thousand, Mw/Mn=3.27) [1] polydispersity were determined, by gel permeation chromatography prior to curing processes in polyblend. For this step the HG-1302 liquid chromatography was used. Three columns filled with Waters® sterogel with different pores. The solvent was chloroform and flow rate and temperature conditions were 1ml/min and 298 K ± 0.1, respectively.The following composition of filled polyblend mixture contains these compounds by weight: SKN-40-30-PVC-100; HCPX-4.0; ABTST-2,0; DTBPM-3,0; ZnO-5,0, ED-5-6,0; naphthenic oil (PN-6)-1,5; carbon black (P324)-50,0. The tested physical properties of low molecular compounds are shown in the table 1.
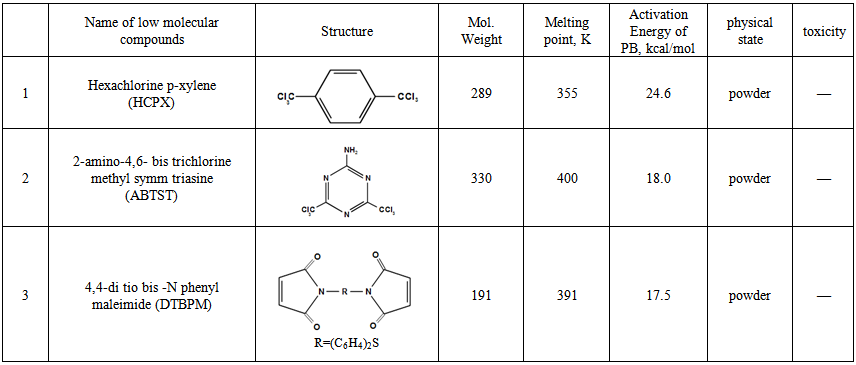 | Table 1. Compound tested as cross-linking (1), sensitizers (2) and stabilizing (3) vulcanization agent for polyblend (SKN-40-PVC-30) |
3. Results and Discussion
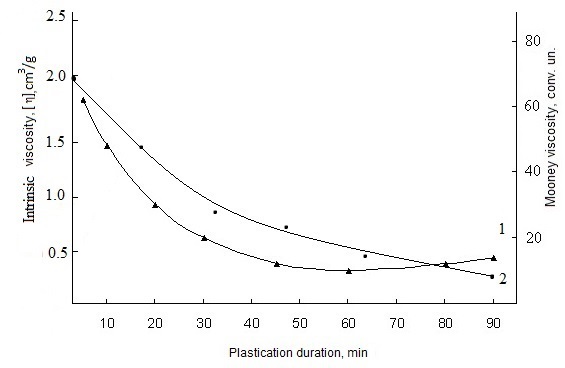
- Cross-linking of polyblend with low-molecular compounds, as a rule, consists of interaction of components of various structure. Basically, any technological characteristics of mixture of butadiene-nitrile rubber with polyvinyl chloride, obviously, will be function of relative content of each component. Therefore, determination of elastic-viscous properties of SKN-40-PVC-30 rubber is an important test which cross-linking of polymer systems should be subjected. The elastic-viscous properties are related with structure and molecular mass during plastification with low-molecular compounds. As the results of investigations, mechanical plastification of mixtures (without technical carbon) is characterized by the change of plasticity, rigidity and Mooney viscosity with the increase of plasticizing time (Figure 1 and 2).It is seen that the insignificant changes of plasticity are observed in mechanical plastification of model mixtures for 20 min. An increase on plasticity time up to 30 min leads to insignificant changes of plasticity parameters and an increase of plastification time up to 90 min deteriorates dramatically. Obviously, this phenomenon is connected with the beginning of destruction time in the samples by the increase of time on mechanical plastification of elastomer mixtures does not lead to essential changes of rigidity coefficient and remains steadily in the ranges between 1000-1200gs (figure 1, curve 2).
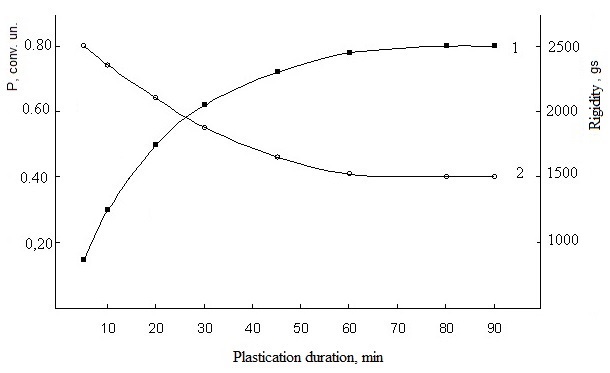 | Figure 1. Dependence of plasticity (1) and rigidity (2) of elastomer mixtures on the basis of SKN-40-PVC-30 on plastification duration |
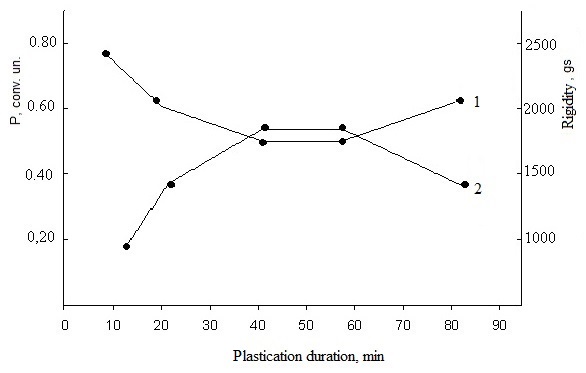 | Figure 3. Dependence of plasticity (1) and rigidity (2) of filled mixtures on the basis of SKN-40- PVC-30 on heating duration (423 K x 90 min) |
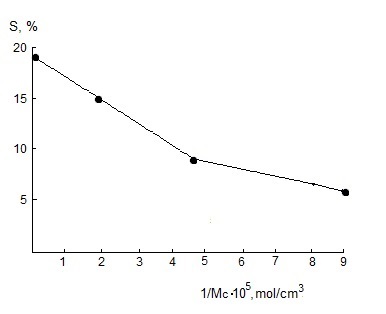 | Figure 4. Dependence of quantity of sol-fraction on concentration of number of chains of networks of filled vulcanizers on the base on SKN-40-PVC-30 |
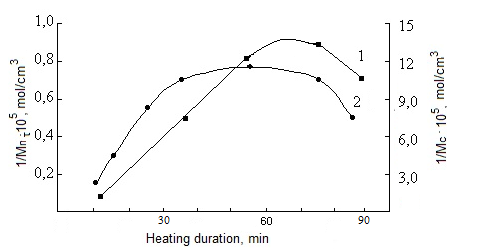 | Figure 5. Dependence of concentration of structure parameters of network of cures on the basis on SKN-40-PVC-30 on heating duration. 1-number of cross-linked molecules, 2-number of chain network |
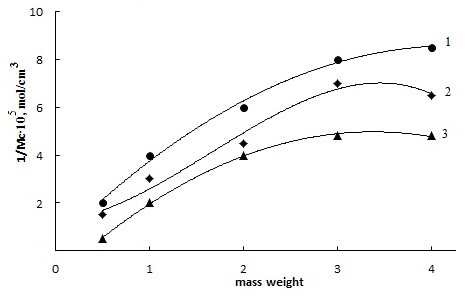 | Figure 6. Influence concentration of HCPX, ABTST and DMPM to the yield of number chain networks on base SKN-40-PVC-30 |
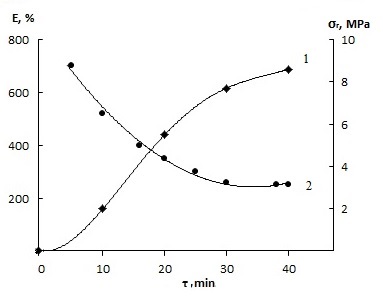 | Figure 7. Influence of time duration to the properties of the cures (SKN-40-PVC-30 -100, HCPX -4,0 + ABTST-2.0 + DTBPM-3, 0) 1-tensile strength; 2- Elongation (mode 423 K × 40') |
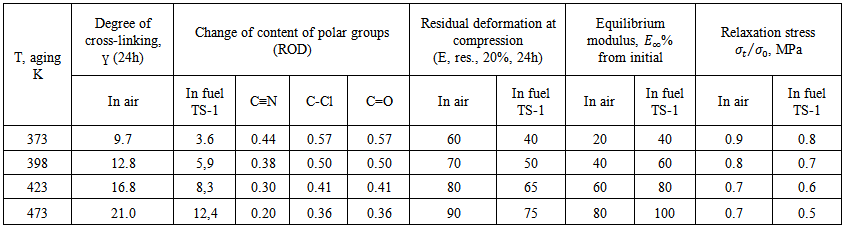 | Table 2. Influence of temperature on aging of elastomer on the base of SKN-40-PVC-30 in air and in fuel |
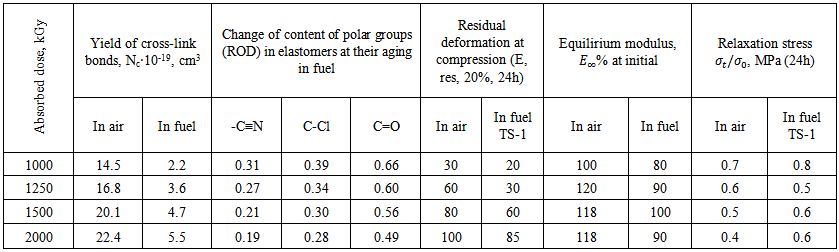 | Table 3. Influence of temperature on aging of elastomer of SKN-40-PVC-30 on air and in fuel |
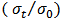 in static conditions of test. According to the GOST-9.701-79 radiation stability of elastomer materials in compressed state is estimated by adsorbed dose at which Eres=80%, and
in static conditions of test. According to the GOST-9.701-79 radiation stability of elastomer materials in compressed state is estimated by adsorbed dose at which Eres=80%, and 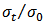 =0.2.
=0.2. 4. Conclusions
- Analysis of the experimental results shows that cross-linking of polyblend which is prepared by emulsion polymerization of low-molecular cross-linking agents, sensitizers and antioxidants (HCPX, ABTST, DTBPM) usually consists of many components of various structures.In principle, any technological characteristics of polyblend obviously will affect the function of the relative content and structural features of each component. This is largely due to the fact that the hyper branched macromolecule characterized by a high density. The most important fact is that the mechanical plastification occurs in a few changes in elastic properties of elastomers. During the processing of polyblend components by mechanical plastification, takes place its failure initial molecular uniformity and the destruction of an ordered structure. Reduction in rigidity during the mechanical plastification can be explained by an increase of elasticity and defects of net.Correction equations theory of high elasticity gave the opportunity to use them mainly for qualitative assessment of the spatial grid density of vulcanizers. The results show that the low molecular weight compounds have structural parameters, thermal vulcanizes accelerate cross-linking yield, and by the cross-linked molecules increases monotonically with increasing cure time.Comparison of the structural features of grids cured products (stapling ) of reservoir with their elastic and physical and chemical properties suggests that in the presence of 4.0 wt. of HCPX, 2.0 wt. of ABTST and 3.0 wt. of DTBPM causes inhibition processes on aging temperature of elastomeric materials in air and in fuel.The presence of polar groups (-C≡N, C=O, C-Cl) provides effective protection elastomer materials in air and in fuel.
ACKNOWLEDGEMENTS
- The authors consider as their duty to express sincere gratitude to the collaborator of the St.-Petersburg Technological State Institute of technique, Prof. Dr. Bogdanov V.V. for kindly panting an opportunity for determination of deformation characteristics of thermal vulcanizers.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML