-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Polymer Science
p-ISSN: 2163-1344 e-ISSN: 2163-1352
2014; 4(2): 25-31
doi:10.5923/j.ajps.20140402.01
Cross-linking in Hydrogels - A Review
Jaya Maitra, Vivek Kumar Shukla
Gautam Buddha University, Greater Noida, Gautam Budh Nagar-201312 (U.P), India
Correspondence to: Jaya Maitra, Gautam Buddha University, Greater Noida, Gautam Budh Nagar-201312 (U.P), India.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Hydrogels represent a class of high water content polymers with physical or chemical crosslinks. Their physical properties are similar to soft tissues. Cross linking is a stabilization process in polymer chemistry which leads to multidimensional extension of polymeric chain resulting in network structure. Cross-link is a bond which links one polymer chain to other. It can be ionic or covalent. Cross linking changes a liquid polymer into ‘solid’ or ‘gel’ by restricting the ability of movement. When polymer chains are linked together by cross-links, they lose some of their ability to move as individual polymer chains. A liquid polymer (where the chains are freely flowing) can be turned into a ‘solid’ or ‘gel’ by cross-linking the chains together. Cross linking increases the molecular mass of a polymer. Cross-linked polymers are important because they are mechanically strong and resistant to heat, wear and attack by solvents. However, the drawback associated with cross-linked polymers is that they are relatively inflexible when it comes to their processing properties because they are insoluble and infusible.
Keywords: Hydrogels, Cross linking, Gel, Polymer
Cite this paper: Jaya Maitra, Vivek Kumar Shukla, Cross-linking in Hydrogels - A Review, American Journal of Polymer Science, Vol. 4 No. 2, 2014, pp. 25-31. doi: 10.5923/j.ajps.20140402.01.
Article Outline
1. Introduction
- Hydrogels are crosslinked hydrophilic polymer structures that can imbibe large amounts of water or biological fluids. Hydrogels are one of the upcoming classes of polymer-based systems that embrace numerous biomedical and pharmaceutical applications. Because of their inherent property of biocompatibility they offer good opportunities as protein delivery systems or tissue engineering scaffolds. Their hydrophilic, soft and rubbery nature ensures minimal tissue irritation and a low tendency of cells and proteins to adhere to the hydrogel surface.The use of hydrogel for biomedical applications dates back to 1960 when Wichterle and Lim developed crosslinked poly (hydroxyethyl methacrylate) (pHEMA) [1]. First synthetic hydrogels of HEMA with EGDMA (Ethylene glycol di-methyl acrylate) as cross-linker were prepared for biological use and later used for production of contact lenses [1].Because of their versatile and unique properties, hydrogels have vast potential applications, including soil/water stabilization layers in farming and civil engineering structures [2], soil conditioners, controlled release of fertilizers [3,4], fiber and metallic cable sealing [5], in water technologies [6], thickening agents for cosmetics [7], in drug delivery systems [8] and in many other fields. One of the most dynamic fields in which the super-absorbent hydrogels play the principle role is in the manufacture of personal care products such as feminine hygiene products, adult incontinence products and disposable diapers [9].
2. Significance of Crosslinking
- Adding cross-links between polymer chains affect the physical properties of the polymer depending upon the degree of cross linking and presence and absence of crystallinity. Cross linking results in:i) Elasticity (they can stretch and return to their original form). Elastomers are elastic polymers created by limited cross-linking. As the number of cross-links increases, however, the polymer becomes more rigid and cannot stretch as much; the polymer will become less viscous and less elastic and might even become brittle.The vulcanization or sulfur curing of rubber, for example, results from the introduction of short chains of sulfur atoms that link the polymer chains in natural rubber. Bridges made by short chains of sulfur atoms tie one chain of polyisoprene to another, until all the chains are joined into one giant super molecule. The chemical process of vulcanization is a type of cross-linking which increases the strength of rubber. It makes rubber hard and durable material associated with car and bike tires. ii) Decrease in the viscosity (the resistance to flow) of polymers. In order for polymers to flow, the chains must move past each other and cross-linking prevents this. As a result of restriction in flow there is improvement in the creep behavior.iii) Insolubility of the polymer. Cross linking results in insolubility as the chains are tied together by strong covalent bonds. Crosslinked materials can't dissolve in solvents, but can absorb solvents. Crosslinked material after absorbing lot of solvent is called a gel. For example crosslinked polyacrylamide gel. Uncrosslinked polyacrylamide is soluble in water, and crosslinked polyacrylamides can absorb water but is insoluble. Water-logged gels of crosslinked polyacrylamides are used to make soft contact lenses. iv) Increased Tg and increase strength and toughness. Crosslinking changes the local molecular packing, resulting decrease in free volume, leading to increase in Tg. PVA crosslinked with boric acid showed increased glass transition temperature [10]. Cross-links slow down the PVA molecular motion and must not be included in the crystalline domains.v) Lower melting point. For crystalline polymer with low degree of cross linking there is a decrease in the crystalline behavior, as cross linking introduces hindrance to the chain orientation resulting in softer, elastic polymer having lower melting point.vi) Transformation of therrmoplasts into thermosets. Heavy cross-linking changes thermoplasts to thermoset plastics. Once the cross-links form, the polymer’s shape cannot be changed again without destroying the plastic. Unlike thermoplastic polymers, the process cannot be undone by reheating; thermoset plastics will start to decompose rather than becoming moldable and pliable. The first thermoset was polyisoprene. More the sulfur crosslinks put into the polyisoprene, the stiffer it gets. Lightly crosslinked, it's a flexible rubber. Heavily crosslinked, becomes a hard thermoset.
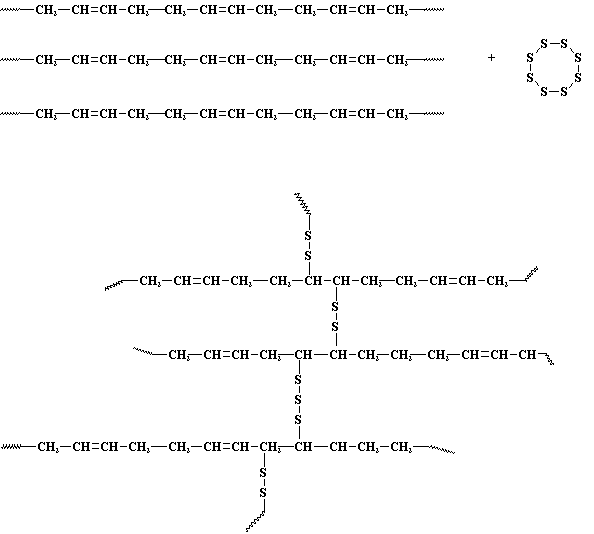 | Figure 1. Vulcanization of rubber |
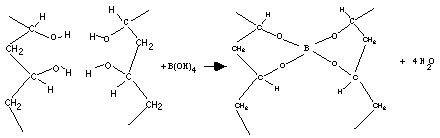 | Figure 2. Reaction of PVA with boric acid |
3. Methods of Crosslinking
- Depending upon the nature of the polymer, different techniques may be used to cause cross linking. Cross-linking may occur through polymerization of monomers having functionalities more than two (by condensation) or by covalent bonding between polymeric chain through irradiation, sulphur vulcanization or chemical reactions by adding different chemicals in conjunction with heating and, sometimes, pressure. In all cases, the chemical structure of the polymer is altered through the cross linking process. Cross linking by irradiation is done by using high-energy ionizing radiation, like electron beam (e-beam), gamma, or x-ray. Gamma irradiation is usually most economical at lower doses (~80 kGy and below) and for large, high density parts. Electron beam is commonly used for small parts, particularly low density parts, and in linear product processed reel to reel (eg, wire, cable, tubing).
4. Hydrogels and Crosslinking
- The term “hydrogel” represent water insoluble polymeric network that has capacity to absorb large amount of water [11-15]. A hydrogel is a macromolecular polymer gel constructed of a network of crosslinked polymer chains. They are synthesized from hydrophlic monomers by either chain or step growth, along with a functional crosslinker to promote network formation. Synthetic or natural polymers, homopolymer or copolymer, are used to make three dimensional networks by molecular entanglements or by chemical crosslinking [16].
 | Figure 3. Cross-linking in polymer |
5. Synthesis of Hydrogels
- Hydrogels are synthesized by different polymerization methods using both chemical and physical crosslinking routes. Both natural polymers such as proteins or synthetic polymers like PVA with a high affinity for water can be crosslinked. Different crosslinking methods can be implemented for the design of a hydrogel. The following chemical and physical methods reflect the synthesis of hydrogels. PVA cross-linked membranes were synthesized using glutaraldehyde as cross-linking agent [63]. Chemically crosslinked hydrogels are synthesized by chain growth polymerization, addition and condensation polymerization and gamma and electron beam polymerization.Chain-growth polymerization includes free radical polymerization, controlled free radical polymerization, anionic and cationic polymerization. It is done by three process viz., initiation, propagation, and termination. After initiation, a free radical active site is generated which adds monomers in a chain link-like fashion.Poly (N-isopropyl acrylamide) hydrogel are synthesized by typical free radical polymerization PVA based hydrogels are prepared by free radical copolymerization. PVA has been cross-linked chemically with monomer (methacrylic acid) in aqueous medium using ethylene glycol di-methacrylate (EGDMA) as cross-linking agent and benzoyl peroxide as reaction initiator. Monomer MAA is used to impart pH sensitive characteristics. This pH sensitive chemically cross-linked PVA hydrogels is a promising delivery system for colonic delivery of 5-fluorouracil in colorectal cancer [64].Controlled living radical polymerizations offer the benefits of longer growing chain life compared to free radical polymerizations for macromolecular engineering. Anionic and Cationic polymerization methods suffer from extreme sensitivity toward aqueous environments and therefore, are not used in the synthesis of polymeric hydrogels.Addition and condensation polymerization involves stepwise addition of Polyfunctional crosslinking agents with monomer functional groups. Water soluble monomers can be converted into hydrogels using crosslinking agents such as tetramethylethylenediamine (TEMED). Polymer chains may be crosslinked in the presence of water to form a hydrogel. Water occupies voids in the network, giving the hydrogel its characteristic surface properties. Polyurethanes, polyesters, or nylon polymers are most commonly synthesized for hydrogel applications [65].Gamma and electron beam polymerization involves high energy electromagnetic irradiation as crosslinker. These high energy radiations can crosslink water-soluble monomer or polymer chain ends without the addition of a crosslinker. During irradiation, using a gamma or electron beam, aqueous solutions of monomers are polymerized to form a hydrogel. Gamma and electron beam polymerizations also involves the initiation, propagation, and termination steps as in the free radical polymerization. Hydroxyl radicals are formed and initiate free radical polymerization among the vinyl monomers which propagate in a rapid chain addition fashion [65]. The hydrogel is finally formed once the network reaches the critical gelation point. This process has an advantage over other crosslinking methods since it can be performed at room temperature and in physiological pH without using toxic and hard to remove crosslinking agents such as potassium persulfate [65].Physically crosslinked hydrogels are synthesized by ionic interaction, crystallization, stereocomplex formation, hydrophobized polysaccharides, protein interaction and hydrogen bond.In ionic interactions, hydrogels can be crosslinked under mild conditions, at room temperature and physiological pH. This process of cross-linking doesnot require presence of ionic groups in the polymer. The use of metallic ions yield stronger hydrogel [65]. For stereocomplex formation, a hydrogel is formed through crosslinking that is formed between lactic acid oligomers of opposite chirality [65]. Hydrophobic interactions results in the polymer to swell and uptake water that forms the hydrogel. Polysaccharides such as chitosan, dextran, pullulan and carboxymethyl curdlan [65] are reported in literature for the preparation of physically crosslinked hydrogels by hydrophobic modification.Protein interaction involves block copolymers that contains repetition of silk-like and elastine-like blocks called ProLastins [65]. These ProLastins are fluid solutions in water and can undergo a transformation from solution to gel under physiological conditions because of the crystallization of the silk-like domains [65].Poly Acrylic Acid (PAA) and Poly Methacrylic Acid (PMA) form complexes with Poly Ethylene Glycol (PEG) from the hydrogen bonds between the oxygen of the PEG and carboxylic group of PMA [65]. This interaction allows for the complex to absorb liquids and swell at low pH which transforms the system into a gel. Crystallization involves freezing-thawing process and creates a strong and highly elastic gel [66]. PVA hydrogels can be formed by physically crosslinking through repeated freezing/thawing methods, or chemically crosslinked with glutaraldehyde or epichlorohydrin. Table 1 shows the name of some hydrogels, cross-linking agents and their applications.
6. Conclusions
- Chemical cross-linking is a highly versatile method to improve the mechanical property of the hydrogels. However, cross-linking agents are often toxic compounds and not environmental friendly. They give unwanted reactions with the bioactive substances present in the hydrogel matrix. The adverse effects of chemical cross-linking can be avoided by the process of physical cross linking using radiation or electron beam method. Radiation cross-linking is more advantageous as the amount of cross-linking can be controlled by the amount of dose used and is an energy efficient and cleaner process with no unwanted residuals in the products.
References
| [1] | Wichterle O, Lim D. Hydrophilic gels in biologic use. Nature 1960; 185:117. |
| [2] | Jensen, OM, Hansen, P.F. (2002) Cement Concr. Res., 32: 973–978. |
| [3] | Karadag ED, Saraydin C¸ AldiranY, Gu¨ven,O. (2000) Polym. Adv. Technol., 11: 59–68. |
| [4] | Chen P, Zhang W, Luo W, Fang, Y. (2004) J. Appl. Polym. Sci., 93: 1748–1755. |
| [5] | Sun X, Zhang G, Shi Q, Tang B,Wu Z. (2002) J. Appl. Polym. Sci., 86: 3712–3717. |
| [6] | Chauhan, G.S. and Lal, H. (2003) Desalination, 159: 131–138. |
| [7] | Kulicket W, Kull AH, Kull W, Thielking, Engelhardt HJ, Pannek J. (1996) Polymer 37: 2723–2730. |
| [8] | Chen J, Blevins WE, Park H, Park, K. (2000) J. Controlled Release, 64: 39–51. |
| [9] | Bucholz FL, Graham T. (1998) Modern Superabsorbent Polymer Technology. Wiley-VCH: New York, 1–154. |
| [10] | Tsukasa M , Yuuki T, Sachiko A, Takahiko I, Akie H, Keiko E. Role of boric acid for a poly (vinyl alcohol) film as a cross-linking agent: Melting behaviors of the films with boric acid Polymer Volume 51, Issue 23, 29 October 2010, Pages 5539–5549 |
| [11] | Nguyen KT, West JL. Photopolymerizable hydrogels for tissue engineering applications. Biomaterial 2002, 23:4307-4314.  |
| [12] | Peppas NA, Bures P, Leobandung W, Ichikawa H. Hydrogels in pharmaceutical formulations Eur J Pharm Biopharm 2000, 50:27-46. |
| [13] | Sawhney AS, Pathak CP, van Rensburg JJ, Dunn RC, Hubbell JA. Optimization of photopolymerized bioerodible hydrogel properties for adhesion prevention J Biomed Mat Res 1994, 28:831-838. |
| [14] | Kopecek J. Hydrogel biomaterials: a smart future? Biomaterials. 2007; 28(34):5185–5192. |
| [15] | Lutolf MP. Biomaterials: Spotlight on hydrogels. Nat Mater. 2009;8(6):451–453. |
| [16] | Chung HK, Park TG. Self-assembled and nanostructured hydrogels for drug delivery and tissue engineering. Nano Today. 2009;4(5):429–437. |
| [17] | Oh JK. Engineering of nanometer-sized cross-linked hydrogels for biomedical applications. Can J Chem. 2010; 88(3):173–184. |
| [18] | Ulijn RV, Bibi N, Jayawarna V, et al. Bioresponsive hydrogels. Mater Today. 2007; 10(4):40–48. |
| [19] | Lee J, Cuddihy MJ, Kotov NA. Three-dimensional cell culture matrices: state of the art. Tissue Eng Part B. 2008;14(1):61–86. |
| [20] | Geckil H, Xu F, Zhang XH, Moon S, Demirici U. Engineering hydrogels as extracellular matrix mimics. Nanomedicine. 2010;5(3):469–484. |
| [21] | Hunt NC, Grover LM. Cell encapsulation using biopolymer gels for regenerative medicine. Biotechnol Lett. 2010;32(6): 733–742. |
| [22] | Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24(24):4337–4351. |
| [23] | Liu SQ, Tay R, Khan M, Ee PLR, Hedrick JL, Yang YY. Synthetic hydrogels for controlled stem cell differentiation. Soft Matter. 2010;6(1):67–81. |
| [24] | Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA. Hydrogels in regenerative medicine. Adv Mater. 2009;21(32–33):3307–3329. |
| [25] | Nicodemus GD, Bryant SJ. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng Part B Rev. 2008;14(2):149–165. |
| [26] | Cushing MC, Anseth KS. Hydrogel cell culture. Science. 2007;316(5828):1133–1134. |
| [27] | Miyata T, Uragami T, Nakamae K: Biomolecule-sensitive hydrogels Adv Drug Deliv Rev 2002, 54:79-98. |
| [28] | Chang C, Duan B, Cai J, Zhang L: Superabsorbent hydrogels based on cellulose for smart swelling and controllable delivery, Eur Polym J. 2010;21:92–100. |
| [29] | Nguyen TK, West JL. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials. 2002;23(22): 4307–4314. |
| [30] | Aly A.S. (1998). Self-dissolving chitosan. I. Preparation and characterisation and evaluation for drug delivery system. Angew Macromolecular Chemistry. 259, 33-38. |
| [31] | Jack J. Connell (1975). The role of formaldehyde as a protein crosslinking agent acting during the frozen storage of cod. J. Sci. Food and Agriculture. 26(12), 1925-1929. |
| [32] | Imamura E, Sawatani O, Koyanagi H, NoishikiY, and Miyata T, (1989). “Epoxy Compounds As a New Cross-Linking Agent for Porcine Aortic Leaflets: Subcutaneous Implant Studies in Rats.” Journal of Cardiac Surgery, 4: 50–57. |
| [33] | Gulati. N, Nagaich. , Sharma. V.K, and Khosa. R.L (2011). “Effect of Polymer and Cross Linking Agent on In Vitro Release of Quercetin from Microbeads” Asian Journal of Pharmacy and Life Science. 1(4): 2231 – 4423. |
| [34] | Zhu J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials. 2010;31(17): 4639–4656. |
| [35] | Ramamurthi A, Vesely I, Ultraviolet light-induced modification of crosslinked hyaluronan gels. J Biomed Mater Res A. 2003; 66(2):317–329. |
| [36] | Denizli BK, Can HK, Rzaev ZMO, Guner A. Preparation conditions and swelling equilibria of dextran hydrogels prepared by some crosslinked agents. Polymer. 2004;45(19): 6431–6435. |
| [37] | Kuo CK, Ma PX. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: part I. Structure, gelation rate and mechanical properties. Biomaterials. 2001;22(6): 511–521. |
| [38] | Kim IY, Seo SJ, Moon HS, et al. Chitosan and its derivatives for tissue engineering applications. Biotechnol Adv. 2008; 26(1):1–21. |
| [39] | Liang Y, Liu W, Han B, et al. An in situ formed biodegradable hydrogel for reconstruction of the corneal endothelium. Coll Surf B. 2011;82(1):1–7. |
| [40] | Davidenko N, Campbell JJ, Thian ES, Watson CJ, Cameron RE. Collagen-hyaluronic acid scaffolds for adipose tissue engineering. Acta Biomater. 2010;6(10):3957–3968. |
| [41] | Stabenfeldt SE, Garcia AJ, LaPlaca MC. Thermoreversible laminin-functionalized hydrogel for neural tissue engineering. J Biomed Mater Res A. 2006;77(4):718–725. |
| [42] | Shikanov A, Xu M, Woodruff TK, Shea LD. Interpenetrating fibrin-alginate matrices for in vitro ovarian follicle development. Biomaterials. 2009;30(29):5476–5485. |
| [43] | Sakai S, Hashimoto I, Kawakami K. Synthesis of an agarose-gelatin conjugate for use as a tissue engineering scaffold. J Biosci Bioeng. 2007;103(10):22–26. |
| [44] | Huang Y, Onyeri S, Siewe M, Moshfeghian A, Madihally SV. In vitro characterization of chitosan–gelatin scaffolds for tissue engineering. Biomaterials. 2005;26(36):7616–7627. |
| [45] | Dhandayuthapani B, Krishnan UM, Sethuraman S. Fabrication and characterization of chitosan-gelatin blend nanofiber for skin tissue engineering. J Biomed Mater Res B Appl Biomater. 2010;94(1):264–272. |
| [46] | Tan H, Wu J, Lao L, Gao C. Gelatin/chitosan/hyaluronan scaffold intergrated with PLGA microspheres for cartilage tissue engineering. Acta Biomater. 2009;5(1):328–337. |
| [47] | Rosellini E, Cristallini C, Barbani N, Vozzi G, Giusti P. Preparation and characterization of alginate/gelatin blend films for cardiac tissue engineering. J Biomed Mater Res A. 2009;91(2):447–453. |
| [48] | Liu Y, Chan-Park MB. Hydrogel based on interpenetrating polymer networks of dextran and gelatin for vascular tissue engineering. Biomaterials. 2009;30(2):196–207. |
| [49] | Hoffman AS. Hydrogels for biomedical applications. Adv Drug Deliver Rev. 2002;43(1):3–12. |
| [50] | Lin CC, Metters AT. Hydrogels in controlled release formulations: network design and mathematical modeling. Adv Drug Deliver Rev. 2006;58(12–13):1379–1408. |
| [51] | Schneider GB, English A, Abraham M, Zaharias R, Stanford C, Keller J. The effect of hydrogel charge density on cell attachment. Biomaterials. 2004;25(15):3023–3028. |
| [52] | Hejcl A, Sedy J, Kapcalova M, et al. HPMA-RGD hydrogels seeded with mesenchymal stem cells improve functional outcome in chronic spinal cord injury. Stem Cells Delvelop. 2010;19(10):1535–1546. |
| [53] | Woerly S, Pinet E, de Robertis P, Van Diep D, Bousmina M. Spinal cord repair with PHPMA hydrogel containing RGD peptide (NeuroGel™) Biomaterials. 2001;22(10):1095–1111. |
| [54] | Takezawa T, Mori Y, Yoshizato K. Cell culture on a thermo-responsive polymer surface. Nat Biotechnol. 1990;8(9):854–856. |
| [55] | Vihola H, Laukkanen A, Valtola L, Tenhu H, Hirvonen J. Cytotoxicity of thermosensitive polymers poly (N-isopropylacrylamide), poly(N-vinylcaprolactam) and amphiphilically modified poly(N-vinylcaprolactam) Biomaterials. 2005;26(16):3055–3064. |
| [56] | Park KH. Improved long-term culture of hepatocytes in a hydrogel containing Arg-Gly-Asp (RGD) Biotechnol Lett. 2002;24(14):1131–1135. |
| [57] | Buxton AN, Zhu J, Marchant RE, West JL, Yoo JU, Johnstone B. Design and characterization of poly(ethylene glycol) photopolymerizable semi-interpenetrating networks for chondrogenesis of human mesenchymal stem cells. Tissue Eng. 2007;13(10):2549–2560. |
| [58] | Beamish JA, Zhu J, Kottke-Marchant K, Marchant RE. The effects of monoacrylate poly(ethylene glycol) on the properties of poly(ethylene glycol) diacrylate hydrogels used for tissue engineering. J Biomed Mater Res A. 2010; 92(2):441–450. |
| [59] | Yang F, Williams CG, Wang D, Lee H, Manson PN, Elisseeff J. The effect of incorporating RGD adhesive peptide in polyethylene glycol diacrylate hydrogel on osteogenesis of bone marrow stromal cells. Biomaterials. 2005;26(30): 5991–5998. |
| [60] | Ossipov DA, Brannvall K, Forsberg-Nilsson K, Hilborn J. Formation of the first injectable poly(vinyl alcohol) hydrogel by mixing of functional PVA precursors. J Appl Polym Sci. 2007;106(1):60–70. |
| [61] | Sawhney AS, Pathak CP, Hubbell JA. Bioerodible hydrogels based on photopolymerized poly(ethylene glycol)-co-poly (α-hydroxy acid) diacrylate macromers. Macromolecules. 1993;26(4):581–587. |
| [62] | Gibas I, Janik H. Review: synthetic polymer hydrogels for biomedical applications Chemistry Chemical technol 2010, 4:297-304. |
| [63] | Alemzadeh I, Vossoughi M.Controlled release of paraquat from poly vinyl alcohol hydrogel. Chem Eng Process 2002, 41:707-710 |
| [64] | M. Minhas, M. Ahmad, L. Ali and M. Sohail Synthesis of chemically cross-linked polyvinyl alcohol-co-poly (methacrylic acid) hydrogels by copolymerization; a potential graft-polymeric carrier for oral delivery of 5-fluorouracil DARU Journal of Pharmaceutical Sciences 2013, 21:44. |
| [65] | Hennink,W.E., Nostrum, C.F. van. Department of Pharmaceutics, Utrecht University. Advanced Drug Deliveries Review. Volume 54, January 2002, 13-36. |
| [66] | Yokoyama F, Masada I, Shimamura K, Ikawa T, Monobe K. Morphology and structure of highly elastic poly(vinyl alcohol) hydrogel prepared by repeated freezing-and-melting Colloid Polym. Sci., 264 (1986), pp. 595–601. |
| [67] | Gómez-Mascaraque LG, Méndez JA, Fernández-Gutiérrez M, Vázquez B, San Román J Oxidized dextrins as alternative crosslinking agents for polysaccharides: application to hydrogels of agarose-chitosan. Acta Biomater. 2014 Feb;10(2):798-811. |
| [68] | Kawase M, Michibayashi N, Nakashima Y, Kurikawa N, Yagi K, Mizoguchi T. Application of glutaraldehyde- crosslinked chitosan as a scaffold for hepatocyte attachment. Biol Pharm Bull. 1997 Jun;20(6):708-10. |
| [69] | Tang Hongbo, Li Yanping, Sun Min and Wang Xiguang Preparation and property of crosslinking guar gum Polymer Journal (2012) 44, 211–216. |
| [70] | López-Cebral R, Paolicelli P, Romero-Caamaño V, Seijo B, Casadei MA, Sanchez ASpermidine-cross-linked hydrogels as novel potential platforms for pharmaceutical applications J Pharm Sci. 2013 Aug;102(8):2632-43. |
| [71] | Xu Xu, Yuhua Weng, Lu Xu, Hao Chen Sustained release of avastin® from polysaccharides cross-linked hydrogels for ocular drug delivery International Journal of Biological Macromolecules Volume 60, September 2013, Pages 272–276. |
| [72] | Raut NS, Deshmukh PR, Umekar MJ, Kotagale NR Zinc cross-linked hydroxamated alginates for pulsed drug release Int J Pharm Investig. 2013 Oct;3(4):194-202. |
| [73] | Taha MO, Nasser W, Ardakani A, Alkhatib HS. Sodium lauryl sulfate impedes drug release from zinc-crosslinked alginate beads: switching from enteric coating release into biphasic profiles Int J Pharm. 2008 Feb 28;350(1-2):291-300. Epub 2007 Sep 14. |
| [74] | Coviello T, Grassi M, Lapasin R, Marino A, Alhaique F Scleroglucan/borax: characterization of a novel hydrogel system suitable for drug delivery. Biomaterials. 2003 Jul;24(16):2789-98. |
| [75] | Hussain T, Ansari M, Ranjha NM, Khan IU, Shahzad Y. Chemically cross-linked poly(acrylic-co-vinylsulfonic) acid hydrogel for the delivery of isosorbide mononitrate. Scientific World Journal. 2013 Oct 23;2013:340737. |
| [76] | Sherr AE, and Swift AM,Crosslinked polyacrylamide gel as a dehydrating agent. Journal of Applied Polymer Science 99(12): 3929–3934, Dec 65. |
| [77] | Abdel-Halim ES, Al-Deyab SS, Hydrogel from crosslinked polyacrylamide/guar gum graft copolymer for sorption of hexavalent chromium ion Carbohydrate Polymers Volume 86, Issue 3, 30 August 2011, 1306–1312. |
| [78] | Soppirnath KS, Aminabhavi TM. Water transport and drug release study from cross-linked polyacrylamide grafted guar gum hydrogel microspheres for the controlled release application. Eur J Pharm Biopharm. 2002 Jan;53(1):87-98. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML