| [1] | H.F Mark, N.M. Bikales. C.G.Overberger and G.Menges, 1986, Encyclopedia of Polymer Science and Engineering, Wiley Interscience, New York. |
| [2] | Miller, A.; Szafko, J.; Turska, E. 1977, Reactivity ratios for acrylonitrile–vinyl chloroacetate copolymerization systems, Journal of Polymer Science, 15(1), 51–63. |
| [3] | Bajaj, P.; Sen, K.; Hajir, Bahrama, 1996, S. Solution polymerization of acrylonitrile with vinyl acids in dimethylformamide, Journal of Applied Polymer Science, 59(10), 1539–1550. |
| [4] | Soykan, C., Coskun M., and Ahmedzade, M. 2000, Synthesis and characterization of phenacyl methacrylate – acrylonitrile copolymers and determination of monomer reactivity ratios, Polymer International, 49(6), 479–484. |
| [5] | Vogl.O.; Albertsso, A.C.; Janovic,.Z. 1985, New Developments in Speciality Polymers, Polymeric Stabilizers, Polymer. 26(9), 1288-1296. |
| [6] | Radic, D.; Gargallo, L. 1997, Synthesis, Reactivity Ratios, and Solution Behavior of Vinylpyrrolidone-co-Monoalkyl Itaconate and Vinylpyrrolidone-co-Dialkyl Itaconate Copolymers, Macromolecules, 30 (4), 817–825. |
| [7] | Queroz, D.; Vargas, R.R.; Higa, O. Z.; Ribeiro R.R.; Vitolo, M. 2002, Lactam–amide graft copolymers as novel support for enzyme immobilization, Journal of Applied Polymer Science, 84(4), 767–777. |
| [8] | Bajpai, S.K.; Sonkusley, J.2002, Hydrogels for oral drug delivery of peptides: Synthesis and characterization , Journal of Applied Polymer Science, 83(8), 1717–1729 |
| [9] | Beitz, T.; Kotz, J.; Wolf, G.; Lleinpeter, E.; Fribery, S.E.2001, Poly(N-vinyl-2-pyrrolidone) and 1-Octyl-2-pyrrolidinone Modified Ionic micro emulsions, Journal of Colloid and Interface Science, 240(2), 581–589. |
| [10] | Basri, M.; Harun, A.; Ahmad, M.B.; Razak, C.A.N; .Salleh, A.B. 2001, Immobilization of lipase on poly(N-vinyl-2-pyrrolidone-co-styrene) hydrogel, Journal of Applied Polymer Science, 82(6), 1404–1409. |
| [11] | Vijaysekaran, S.; Chirila, T.V.; Hong, Y.; Tahija, S.G.; Dalton, P.D.; Constable, I.J.; McAllister. 1996, Poly(I-vinyl-2-pyrrolidinone) hydrogels as vitreous substitutes: Histopathological evaluation in the animal eye, Journal of Biomaterials Science,7(8), 685-696. |
| [12] | Ranucci, H.; Spagnoli, G.; Sartore, L.; Bignottie F.; Ferruti, P. Synthesis and molecular weight characterization of end-functionalized N-vinyl-2-pyrrolidone oligomers, Macromolecular Chemistry and Physics, 196(3), 763–774. |
| [13] | Lee, H. Y.; Yu, S.A.; Jeong K.H.; Kim, Y. 2007, Poly(vinyl pyrrolidone) conjugated lipid system for the hydrophobic drug delivery, Macromolecular Research, 15(6), 547-552. |
| [14] | Otagiri, M.; Imai, T.; Koinuma, H.; Matsumoro, U. 1989, Spectroscopic study of the interaction of coumarin anticoagulant drugs with polyvinylpyrrolidone, Journal of Pharmaceutical and Biomedical Analysis, 7(8), 929–935. |
| [15] | Mumper, R.J.; .Dugauid, J.G.; Anwer, K.; Barron, M.K.; Nitta, H.; Rolland, A.P. 1996, Polyvinyl Derivatives as Novel Interactive Polymers for Controlled Gene Delivery to Muscle, Pharmaceutical Research, 13(5), 701-709. |
| [16] | Reddy, B.S.S.; Arshady R.; George, M. 1985, Copolymerization of N-vinyl-2-pyrrolidone with2,4,5-trichlorophenyl acrylate and with 2-hydroxyethyl methacrylate: Reactivity ratios and molecular weights, European Polymer Journal, 21(6), 511–515. |
| [17] | Morariu, S.; Hulubei, C. 2006, Radical Copolymerization of Functional N-Substituted Maleimides withN-Vinyl-2-Pyrrolidone, High Performance Polymers, 18, 185-198. |
| [18] | Bauduin, G, ; Boutevin, B.; Belbachir, M.; Meghabar, R. 1995, Determination of Reactivity Ratios in Radical Copolymerization: A Comparison of Methods for a Methacrylate/N-Vinylpyrrolidone System, Macromolecules, 28(6), 1750-1753. |
| [19] | Soundarajan, S.; Reddy, B.S.R. 1991, Glycidyl methacrylate and N-vinyl-2-pyrrolidone copolymers: Synthesis, characterization, and reactivity ratios, Journal of Applied Polymer Science, 43(2), 251-258. |
| [20] | Vijay Kumar, S.; Musturappa, T.E.; Prasannakumar, S.;Mahadevan, K.M.; Sheregara, B.S. 2007, N‐Vinylpyrrolidone and Ethoxyethyl Methacrylate Copolymer: Synthesis, Characterization and Reactivity Ratios, Journal of Macromolecular Science, Part A, 44(11), 1161-1169. |
| [21] | Brar, A.S.;.Rajeev Kumar. 2002, Investigation of microstructure of the N-vinyl-2-pyrrolidone/methyl methacrylate copolymers by NMR spectroscopy, Journal of Applied Polymer Science, 85(6), 1328-1336. |
| [22] | Dilek Solpan; Zeynep Kolge; Murat Torun. 2005, Preparation and Characterization of Poly(N‐Vinylpyrrolidone‐co‐Methacrylic Acid) Hydrogels, Journal of Macromolecular Science, Part A, 42(6), 705-721. |
| [23] | Mitesh.B.Patel; Dharmesh.A.Patel; Arabinda Ray and Rajni.M.Patel, 2003, Microbial screening of copolymers of 2,4-dichlorophenyl methacrylate with N-vinylpyrrolidone: synthesis and characterization, Polymer International, 52(3), 367-372. |
| [24] | Nicolas Gatica; Fernando R. Diaz; Ligia Gargallo; Deodato Radic. 1998, Vinyltrimethylsilane-co-N-vinyl-2-pyrrolidone and vinyltrimethoxysilane-co-N-vinyl-2-pyrrolidonecopolymers Synthesis and reactivity ratios, Polymer Bulletin, 40(6), 707-713. |
| [25] | Kelen, T.; Tudos, F. 1975, Analysis of the Linear Methods for Determining Copolymerization Reactivity Ratios. I. A New Improved Linear Graphic Method, Journal of Macromolecular Science: Part A - Chemistry, 9(1), 1-27. |
| [26] | Kelen, T.; Tudos, F.; Foldes ,B. T.; Turcsanyi, B. 1976, Analysis of Linear Methods for DeterminingCopolymerization Reactivity Ratios. III. Linear Graphic Method for Evaluating Data Obtained at High Conversion Levels, Journal of Macromolecular Science: Part A - Chemistry , 10(8), 1513-1540. |
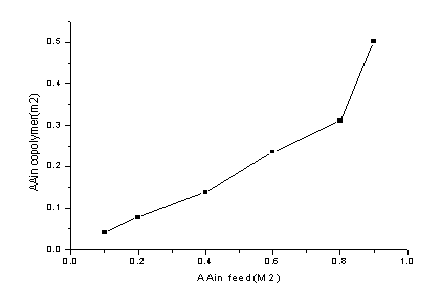
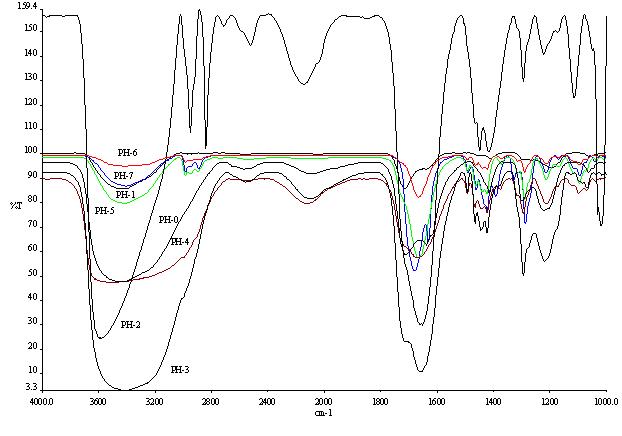
 spectra of the Copolymer
spectra of the Copolymer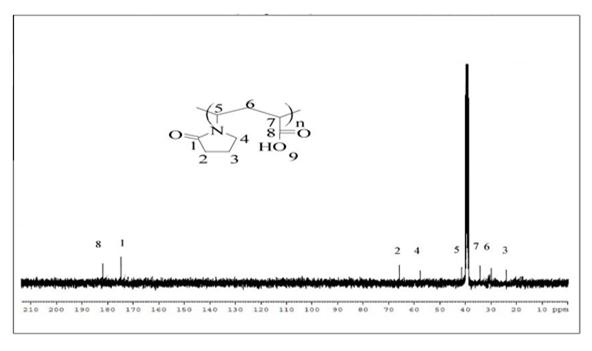
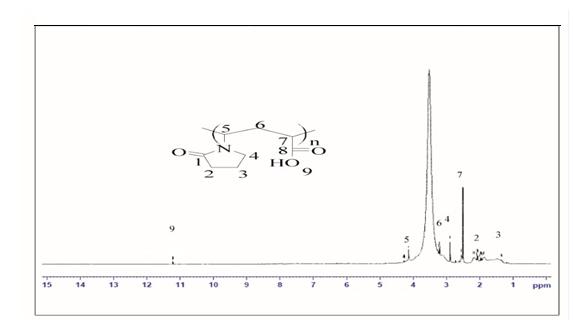
 NMR of Copolymer
NMR of Copolymer
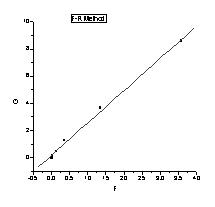
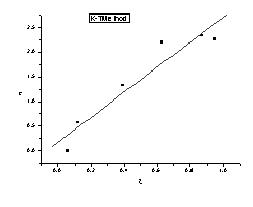
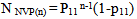
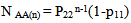
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML