Shiraz M. Mammadov1, Sehrana A. Rzayeva1, Tunzala F. Gojayeva1, Adil A. Garibov1, Ravan N. Mehdiyeva1, Oktay H. Akparov2, Jovdat S. Mammadov1, Elvin M. Aliyev2
1Institute of Radiation Problems of ANAS, Baku, AZ1143, Azerbaijan
2Baku State University, Baku, AZ 1143, Azerbaijan
Correspondence to: Shiraz M. Mammadov, Institute of Radiation Problems of ANAS, Baku, AZ1143, Azerbaijan.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Abstract
The radiation-chemical structure of acrylonitrile-butadiene rubber filled with copolymer vinyl chloride and vinyl acetate in the ratio of 80:20, 75:25, 65:35 has been studied. The changes has been shown by physical-chemical and spectral methods in molecular structure of elastomer with the presence of cross-linking and sensitizing low-molecular organic compounds (disulfochloride benzene and 2,4-diamine-6-phenyl sym triazine) after irradiation dose 100-300 kGy. The radiation and thermal-radiation method of vulcanization in combination of thermal and additional radiation influence has been developed. Radiation-chemical outputs of cross-links, number of cross-linked molecules and active grid chains in elastomers have been defined for each studied system depending on absorbed dose. The technological mode is developed for carrying out thermal-radiation vulcanization of NBR filled with copolymer vinyl acetate and vinyl chloride with the purpose to obtain products, provide high service properties in sea water, petroliferous and mud solutions.
Keywords:
Acrylonitrile-butadiene Rubber, Vulcanization, Copolymer, Vinyl Chloride, Vinyl Acetate, Elastomers, Number of Cross-linked Molecules, Number of Grid Chain, Thermo-radiation, Rheology, Filler
Cite this paper: Shiraz M. Mammadov, Sehrana A. Rzayeva, Tunzala F. Gojayeva, Adil A. Garibov, Ravan N. Mehdiyeva, Oktay H. Akparov, Jovdat S. Mammadov, Elvin M. Aliyev, Radiation-Chemical Structure of Acrylo-nitrile Butadiene Rubber with Copolymer Vinyl Chloride and Vinyl Acetate, American Journal of Polymer Science, Vol. 3 No. 4, 2013, pp. 76-82. doi: 10.5923/j.ajps.20130304.03.
1. Introduction
It is known, that radiation cross-linking process and elastomeric properties are significantly influenced by chemical constitution of macromolecules in the presence of low-molecular components (in particular cross-linking sensitizers)[1-10]. Use of ionizing radiation as a method of structure formation in elastomers opens new possibilities for creating elastomeric materials (EM) with improved service properties. In addition, the elastomeric materials are used in difficult conditions of influence on their aggressive medium in some industries.For changing interfacial interaction of acrylonitrile - butadiene rubber (NBR) with polyfunctional monomers (PFM), and also for supporting the given physical - mechanical and service characteristics of EM, different cross - linking ways which contains bonds between carbon atoms are used in the system[11-15]. The creation and obtaining of aggressively resistant EM by radiation technology method based on NBR, filled with copolymer vinyl chloride (VC) and vinyl acetate (VA), containing active low-molecular compounds and study of some factors influencing on the parameters of spatial grid and also on the physical and mechanical properties of NBR had received little attention. In literature[10-12] few details of controversial character are known only for rubber brands SKN-18, SKN -26 и SKN -40. The Current work presents study of radiation cross-linking process of NBR filled with copolymer VC and VA and in presence of cross-linking agent disulfochloride benzene (DSCB), triazine (TAC) and polyester compounds (PEC), representing considerable interest in view of their high efficiency in linking, sensitizing and plasticizing role in cross-linking process.
2. Experimental
The compositions based on acrylonitrile-butadiene rubber brand SKN-40 filled with copolymer VC and VA has been used for these experiments.The compositions were obtained by mixing rubber latexes and copolymer in relation 80:20, 75:25, 65:35. Mixture was coagulated with aluminum salt at temperature of 310-352К. The formed polymer crumb was washed with water and dried at 343-352К. Dissolution of cross-linking system in rubber was carried out with the method of mechanical mill plasticization. The compositions on 100 parts of rubber were prepared with coupling and sensitizing agent of disulfochloride benzene and 2,4-diamin-6-phenyl sym triazine (DAPST) after thorough mixing within 3-5 min. with friction f=1:2. Property and contents of used linking and sensitizing compounds with known ingredients (1) based on NBR and filled with copolymer VC and VA are given in table 1 and 2.The radiolysis and radiation vulcanization of binary mixtures was carried out by Co60 γ-rays at dose rate 6,9 Gy/s. Calculation of dose absorbed by the studied object was carried out on a technique[22]. Samples weighing 1 and 50g were placed in glass ampoules (“Beam”) and molds. Radiation vulcanization was carried out by Со60 gamma rays[23].Radiation-thermal (thermo radiation) vulcanized rubber and molded rubber parts were obtained from them by pre-heating in the press 453K within 5 min. and subsequent irradiation in air at 303K in the range of 100-500 kGy absorbed doses.For the quantitative analysis of spatial grid, 1/Ms and 1/Mτ were defined according to the number of grid chains and number of cross-linked molecules in elastomer by sol-gel analysis based on sequential extraction of samples at first with acetone and then toluene. Magnitude of 1/Ms and 1/Mτ were calculated on Flory-Rehner equation[18].The changes in molecular structure of cross-linking systems during structuring were judged on IR spectra. Identification of spectra was carried out in accordance correlation tables[19-21].Physical and mechanical properties of the filled samples was defined on samples, obtained at high temperatures, within irradiation process, combined by thermal-radiation way. Table 1. Property of low-molecular cross-linking and sensitizing compounds in radiation-chemical processes
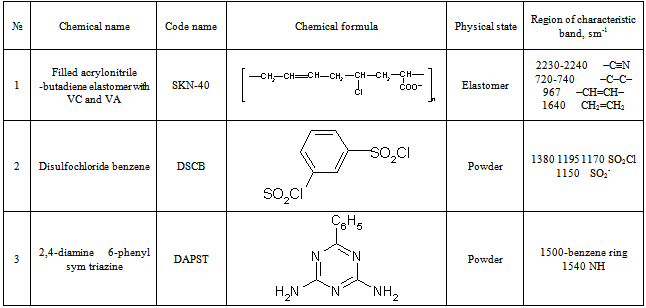 |
| |
|
Table 2. Main ingredients and their contents (w.h.) in elastomeric mixtures on the base of acrylo-nitrile butadiene rubber (SKN-40) filled with copolymer vinyl chloride and vinyl acetate
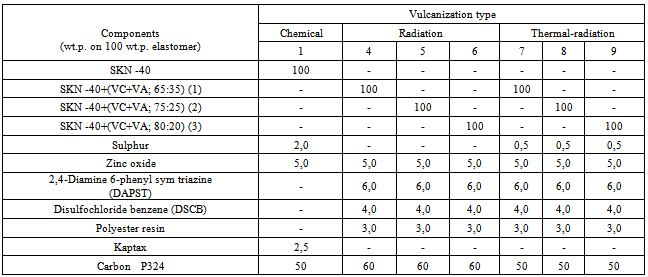 |
| |
|
3. Results and Discussion
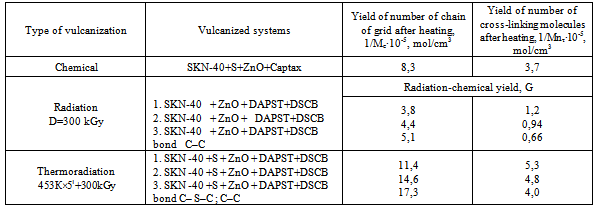
In the process of elastomer mixture irradiation, DSCB and DAPST, which are actively involved in radiation-chemical processes, play an important role as cross-linking and sensitizing agents, and also for reducing absorbed dose.As per the research results at radiation-chemical processes the interaction of low-molecular compounds, filled with NBR copolymer, leading to the formation of a three - dimensional grid, shows the increasing number of chain grid (table 3) in accordance with copolymer content in elastomer and their activity. As per approving views in literature[2] it is assumed that, cross-linking of NBR filled with copolymer at radiation vulcanization arises due to active chlorine atom and carboxyl groups. Quantity of content of copolymers VC with VA in NBR is not the same (80:20, 75:25, 65:35). It should be expected that they will be stitched at different speeds not identical cross-linking level, which is actually observed. The studied elastomers have different speed of cross-links yield (G), which is given in the example at absorbed dose of100-500 KGy (table 3).| Table 3. Radiation-chemical yield of grid chain number (1/Ms) and number of cross-linked molecules (1/Mnτ) in NBR filled with VC copolymer with VA vulcanizated by radiation and thermal-radiation influence |
| | Vulcanizationtype | System | Radiation-chemicalyield, G | | Number of grid chains 1/Ms, mol/sm3 | Number of cross-linked molecules 1/Мnτ, mol/см3 | | RadiationD=300kGy | 1 | 3,8 | 1,2 | | 2 | 4,4 | 0,94 | | 3 | 5,1 | 0,66 | | Thermal-radiation(453К×5`+300kGy) | 1 | 11,4 | 5,3 | | 2 | 14,6 | 4,8 | | 3 | 17,3 | 4,0 |
|
|
Elementary calculations show that at the specified content of VC copolymer with VA the formation of efficient cross-link at 100kGy doses occurs with low speeds. Further, this speed increases with a dose in the ratio 80:20, 75:25 and 65:35.(figure 1) 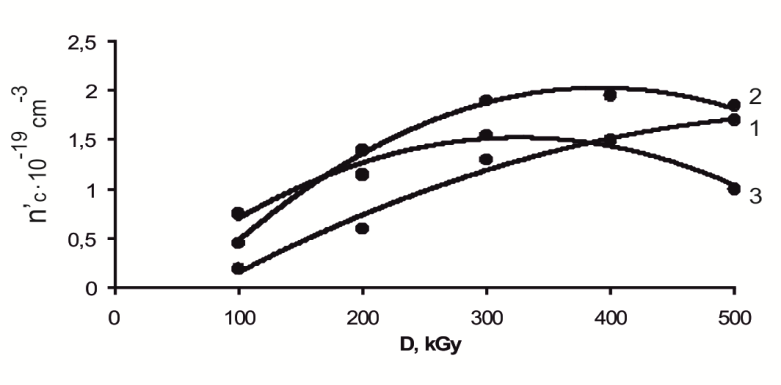 | Figure 1. Kinetics of cross-linking (n`c 1Kinetics of cross-linking (n`c) in structuring of NBR filled with VC and VA at ratio 80:20 (1) 75:25 (2) 65:35 (3) by influence of the absorbed dose (100-500kGy) |
At the dose 400 kGy radiation-chemical yield of number of cross-links in ratio 80:20 of copolymer/ NBR is 1,6∙10-19sm-3, and in ratio 75:25 and 65:35 are 2,1∙10-19 and 1,5∙10-19sm-3. Therefore, cross-linking was carried out not only due to unsaturation of elastomer, apparently vinyl and carboxyl groups or other reactive centers which probably formed primary processes (molecule ionization) in radiation vulcanization process are also involved in crosslinking. The role of influence of joint cross-linking agents of DSCB and sensitizing agent of DAPST in radiation - chemical crosslinking processes has been studied for obtaining optimum properties of elastomer and reducing of absorbed dose in cross-linking process.Analysis of data shows that in the presence of DSCB and DAPST, some changes are observed in cross-link speed (figure 2). So in systems 1 and 3, vulcanized under the influence of irradiation in the presence of DSCB and DAPST the maximum value ns is lower than in the system 3. It is explained by a right ratio choice of VA and VC in copolymer. It should be noted that with increasing irradiation dose, the number of cross-link yields in the filled NBR with sensitizers is 3-4 times higher than in the absence of DSCB and DAPST.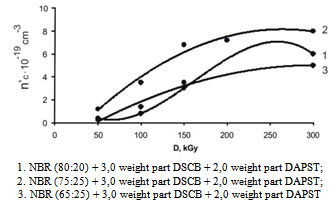 | Figure 2. Kinetics of cross-linking (n`c) in elastomer filled with VC and VA with participation of DSCB and DAPST depend on the absorbed dose |
Thus, radiation yields of elastomer cross-linking processes, containing low-molecular compounds, are caused by percolation of chain radiation-chemical processes highly dependent on dose and polarity of groups, being a part of sensitizer, with percolation of radical reactions. Proceeding from the dependence of radiation vulcanized rubbers properties on ratio value of VC and VA in copolymer, obtain of elastomer with plasticity, providing its technical and technological properties is an important task. It is known that plasticity of NBR (SKN-40) can be adjusted over a wide range as a ready products after irradiation and by mechanical kneading[14]. Varying the content of VC and VA at ratio 80:20, 65:35 in copolymer within the duration of mixture processing on roller (30 min), destruction products of polymer chains with a various lattice density were obtained which negatively influences on plastic-elastic properties of elastomer. Thus, further, ratio 75:25 was used for studying technological properties of NBR filled with copolymer VC and VA.An intense crosslinking on carbon-carbonic bond occurs under the influence of γ-irradiation at a dose of 150-200 kGy and up to 300 kGy these connections practically do not collapse (figure 3 and 4).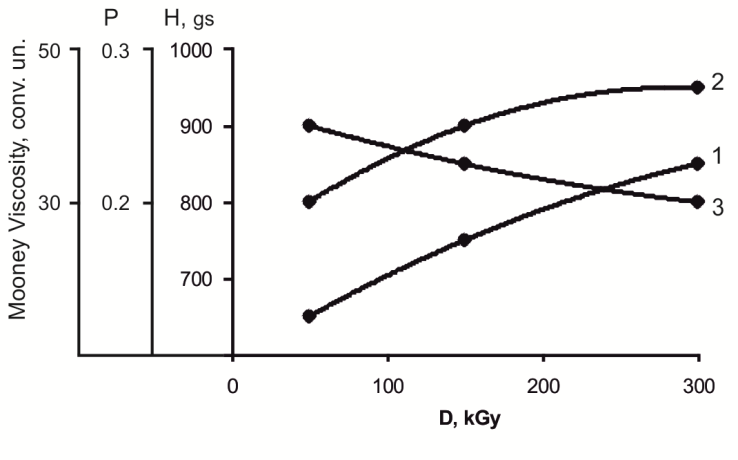 | Figure 3. Influence of irradiation to the plastic and elastic properties of acrylonitrile-butadiene rubber filled copolymer vinyl chloride and vinyl acetate in ratio 75:25. 1-hardness; 2-plasticity; 3-Mooney viscosity |
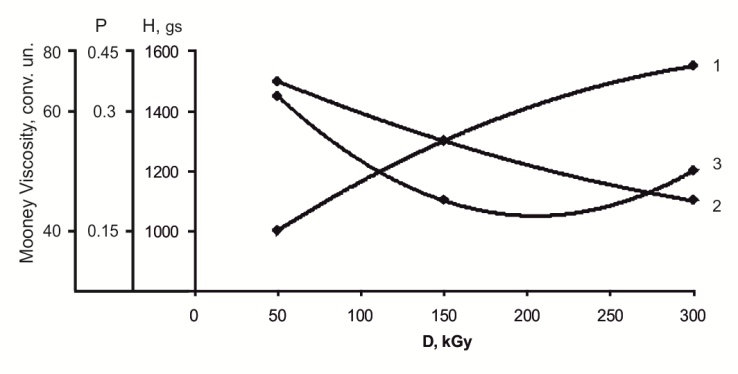 | Figure 4. Influence of thermo-radiation to the plastic and elastic properties of acrylonitrile-butadiene rubber filled copolymer vinyl chloride and vinyl acetate in ratio 75:25. 1-hardness; 2-plasticity; 3-Mooney viscosity |
From the achieved results on change of plasticity, rigidity and Mooney viscosity, within the process of the irradiated samples’ processing on roller, it is seen that plasticity decrease is associated with the destruction of elastomer chain, leading to a change in lattice density, which is bound to an increase in Mooney viscosity, further the rigidity becomes constant.The nature of radiation cross-linking of DSCB filled with copolymers VC and VA (75:25) in the presence of zinc oxide was assessed by IR-spectra changes (figure 5). After irradiation at a dose of 300kGy, a decrease in intensity of absorption bands occurs at 1380, 1195 and 1170 sm-1, characteristic for –SO2Cl groups[19-21], and also a decrease in intensity of absorption bands at 1650sm-1, characteristic for oscillation of –НС=СН– bonds. For the irradiated NBR systems with a copolymer (75:25) + DSCB + ZnO some decrease is observed in optical density of absorption bands at 970 sm-1 characteristic for –НС=СН– double bonds. On the basis of IR-spectra it can be concluded that after system irradiation by splitting off available chlorine in sulfochloride groups, a radiation-chemical interaction of crosslinking agent with double bonds of polymer occurs. Cross-links which formed in this process contain –SO2– sulfonic groups.Tertiary carbon atoms, being one of the butadiene parts of structures 1, 2, can participate in radiation cross-linking reactions of the filled NBR in the presence of DSCB without DAPST. According to literary data, the quantity of links of 1, 2 in SKN-40 elastomer is 10%[1]. Therefore, it should be recognized that defining influence on formation of cross-linked molecules’ yield (1/Мnτ) and number of chain grids (1/Mc) is exerted by tertiary carbon atoms.Thus, on the basis of the conducted researches it is possible to assume the following reaction scheme of filled NBR cross-linking by means of DSCB:It is known that radiation vulcanized rubbers of NBR possess very low strength, so that their use does not present technical interest. As a result of this, the influence of active metal oxides on physical and mechanical properties of vulcanized rubbers should be studied expediently.In Table 4 the corresponding data is shown for the NBR filled with copolymer (at ratio 75:25). As it is seen from the provided data, under optimum conditions zinc oxide activates the cross-linking process and has strength indexes 20-30% higher, including yield and number of stitched molecules.Table 4. Radiation-chemical yield (G), number of chain grid (1/Мс) and number of cross-linking molecules (1/Мnτ) to the one cross-link of elastomer SKN- 40 filled with copolymer vinyl chloride and vinyl acetate (1-3) vulcanizated by radiation-chemical and thermoradiation influence
 |
| |
|
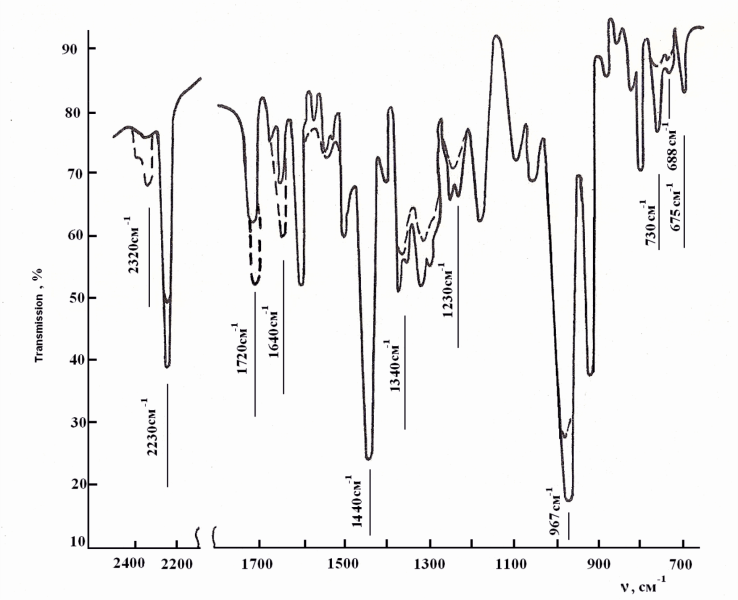 | Figure 5. IR-spectra of acrylonitrile-butadiene rubber filled with copolymer vinyl chloride and vinyl acetate in ratio 75:25 after irradiation by dosage 300kGy; — before irradiation ; after irradiation |
Table 5. Properties of vulcanizates based on NBR filled with copolymer vinyl chloride and vinyl acetate
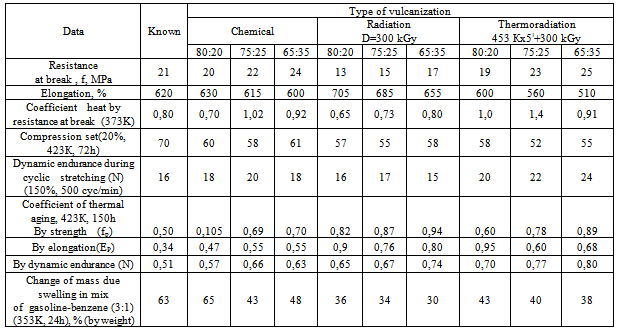 |
| |
|
Introduction of zinc oxide in the studied dosage range (6,0 wt. p) changes the irradiation dose, required for obtaining optimum properties of vulcanized rubbers. The increase in strength of resistance to swelling of heat ageing coefficient can be explained by the influence of metal oxides on the cross-linking speed of radiation vulcanized rubbers. Zinc oxide is characterized by an increased reactivity in relation to hydrogen chloride and accelerates vulcanization of NBR filled with copolymer. It is known[6] that metal oxides aggressively interact with chlorine in an ionic or radical form. The metal chlorides being formed at the result of reaction, in its turn, can activate vulcanization process[4,5,12], and cause a structure-forming effect. Consequently, the role of metal oxides at radiation structuring of NBR filled with disulfochloride aromatic compounds will be defined on the one hand by chlorine fixation rate, on the other hand by the influence of the formed metal chlorides on elastomer structuring rate.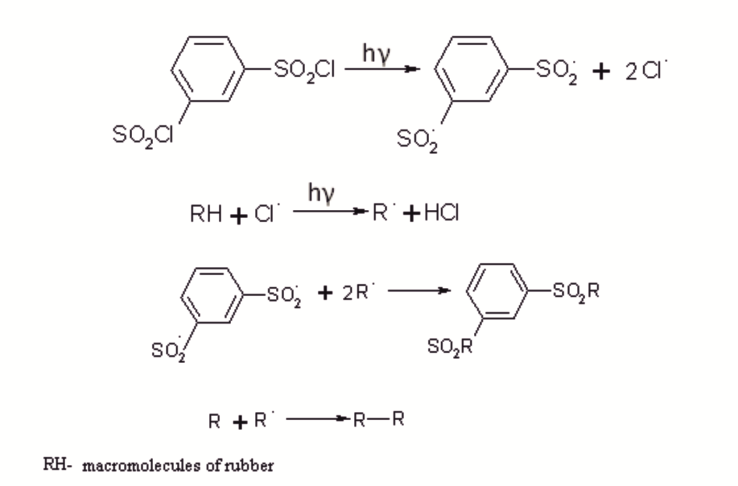 | Scheme 1. Interaction of acrylonitrile-butadiene rubber with disulfo chloride benzene |
It is known that the reduce costs of radiation-vulcanized products – is a development of more economic technology, for example, in[10,11] proposed way of thermal-radiation vulcanization of NBR, which would allow not only to reduce the optimum dose, but also to obtain a product with improved characteristics. Molding products, made by such way, differ by their increased resistance to corrosive medium and their working capacity is 10-15% higher than routine products. We have developed[14-16] a thermal-radiation vulcanization method, the essence of which is the combination of two processes: generally thermal process and additional radiation process. The structure of spatial grid of such vulcanized rubbers differs from both radiation and thermal ones, obtained by heating with sulfur and cross-linking agents.The introduction of 0,2 wt.p sulfur in a mixture, leads to an increase of output of chains grids and cross-linked molecules (table 5), and also physical-mechanical characteristics of thermal-radiation vulcanized rubbers. The increase of physical-mechanical characteristics can be clearly attributed by the formation of polysulfide bonds, along with C–C coupling.It should be noted that thermal-radiation vulcanized rubbers surpass radiation ones, but inferior in resistance to heat ageing and swelling in solvents (table 5). In order to determine the influence of temperature and operation conditions on operating capacity of elastomeric materials, comparative tests of vulcanized rubbers were carried out at 423 K in corrosive medium (table 6) in which pump compactors of drilling rigs and other devices work in oil industry. The achieved data testify that in experimental vulcanized rubbers the swelling level and accumulation of compression set are less and coefficient of heat ageing in sea water and petroliferous solutions is more than in the known chemical vulcanization. Tensile strength after con- tact with medium in radiation vulcanized rubbers was lower than in chemical and thermal-radiation vulcanized rubbers.Table 6. Comparable data of vulcanizates based NBR filled with copolymers vinyl chloride and vinyl acetate in the ratio of 75:25 after exploitation in sea water (I), oil-(II) and clay (III) solutions at 423 K
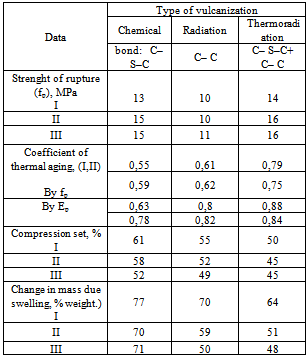 |
| |
|
Thus, analysis of the result of researches allows conclude that the elastomers filled with vinyl chloride and vinyl acetate copolymer with the specified crosslinking systems, obtained in radiation-chemical way, exceed the known ones in resistance to heat ageing and dynamic endurance at repeated stretching in corrosive medium. All studied ingredients are recommended for application in elastomeric materials formulation for preparing sealing parts for boring equipment.
4. Conclusions
The radiation-chemical structuring of acrylonitrile - butadiene rubber filled with copolymer vinyl chloride and vinyl acetate in ratio 80:20, 75:25, 65:35 in the presence of cross-linking and sensitizing agents of DSCB and DAPST has been studied.By sol gel analysis method it was established that the vulcanization of NBR filled with VC copolymer with VA leads to the increase of unsaturation in the NBR molecule and increase of its sensitivity to radiation.The kinetic parameters depending on the velocity and time of vulcanization has been defined for confirmation of the influence of vinyl chloride and vinyl acetate content (80:20, 75:25, 65:35) and low-molecular cross-linking systems on stitching of SKN-40, It is established that the rate of radiation and thermal-radiation structuring of SKN-40, the most active from the studied cross-linking systems is NBR filled with copolymer at ratio 75:25. On the basis of IR-spectroscopy data and rheological analysis, the rate of reaction percolation of the filled rubber SKN-40 between the studied low-molecular compounds (DSCB and DAPST), as well as the influence of gamma irradiation on structure parameters of vulcanized grids is shown. The observations are made about the nature of curing action of the studied structuring systems and features of their influence on rheological and physical-mechanical properties of vulcanized rubbers. The vulcanized rubbers, obtained by radiation and thermal-radiation way are characterized by an average tensile strength, increased coefficient of heat ageing, accumulation of permanent deformation, mass change, and also surpass sulfur vulcanized rubber in resistance to corrosive medium.
ACKNOWLEDGEMENTS
The work has been performed with financial support of the American Foundation “STEP” project №AZC-0905. The authors express their gratitude for the help in carrying out technological researches.
References
| [1] | Mammadov S.M., Yadreyev F.I., Rivin E.M. Butadien - nitrilniye kauchuku i rezini na ix osnove. Baku: Elm, 1991, 280 p. |
| [2] | M. Rojek, J. Stabik, 2006. Modification of PVC compounds with butadiene-acrylonitrile elastomers. J. of Achievements in Materials and Manufacturing Engineering. vo. 17, Is. 1-2, pp 41-48. |
| [3] | Mammadov Sh. M. 2006. Radiasionnaya vulkanizasiya elastomernikh smesey na osnove nasishennogo etilen - propilenovogo kauchuka. Khimicheskiye problemi, №4 pp. 648-652 |
| [4] | Mammadov S.M., Garibov A.A., Salehov A.K., 2009, Troynaya sopolimerizasiya khloroprena, nitrilakrila i dietilovogo efira maleinovoy ksiloti v emulsii. Khimiya i khimicheskaya tekhnologiya. vo.52, is.12, pp.93-99 |
| [5] | S.M. Mamedov, V.Y. Gasanov, G.Z. Velibekova, A.A. Garibov, 2010. Radiolysis of a mixture of butadiene-nitrile rubber with polyvinyl chloride. High Energy Chemistry, vo. 44, Is. 4, pp. 268-271 DOI: 10.1134/S0018143910040028 |
| [6] | Z.Holik, M. Danek, M. Manas, J. Cerny, M. Malachova. 2011 The Influence of Ionizing Radiation on Chemical Resistance of Polymers. Int. J. Of Mechanics. Is. 3, vo. 5, pp. 210-217 |
| [7] | N. A. Shaltout, M. M. Abou Zeid, M. A. Mohamed, A. A. El Miligy, E. M. A. Bary, 2008. Radiation Vulcanization of Nitrile Butadiene Rubber/Butadiene Rubber Blends, J. of Macromol. Sci.; Part A: Pure and Applied Chemistry: vo.1, pp. 225-231. DOI: 10.1080/10601320701842043 |
| [8] | G. Marković, M. Marinović-Cincović , V. Jovanović, S. Samaržija-Jovanović, J. Budinski-Simendić, 2009. The effect of gamma radiation on the ageing of sulfur cured NR/CSM and NBR/CSM rubber blends reinforced by carbon black. Chemical Industry & Chemical Engineering Quarterly 15 (4) pp. 291−298 DOI: 10.2298/CICEQ0904291M |
| [9] | M. Mammadov, A.A. Garibov, O.H. Akperov, S.A. Rzayeva, A.K. Salehov, S.S. Ahmadova 2012. The Influence of γ-Irradiation on Structuration in Solutions of BNR and Properties of Films. Open J. of Phys. Chem., is. 2, pp. 182-184 DOI: 10.4236/ojpc.2012.23024 |
| [10] | M. M. Hassan, R. O Aly, A. El-Ghandour, H. A Abdelnaby, 2013. Effect of gamma irradiation on some properties of reclaimed rubber/nitrile–butadiene rubber blend and its swelling in motor and brake oils. J. of Elastomers and Plastics, vo. 45, no1 pp.77-94. DOI:10.1177/0095244312445523 |
| [11] | T. Yasin, Ahmed, F. Yoshii, K. Makuuchi, 2002, Radiation vulcanization of acrylonitrile–butadiene rubber with polyfunctional monomers. Reactive & Functional Polymers, vo. 53, pp. 173–181. DOI:10.1016/S1381-5148(01)00112-2 |
| [12] | Ping Xiang, Xiu-Ying Zhao, Da-Ling Xiao, Yong-Lai Lu, Li-Qun Zhang, 2008. The Structure and Dynamic Properties of Nitrile–Butadiene Rubber/Poly(vinyl chloride)/Hindered Phenol Crosslinked Composites. J. of Applied Polymer Sci., vo. 109, pp. 106–114 DOI 10.1002/app.27337 |
| [13] | S.M. Mammadov, S.A.Rzayeva, A.A. Garibov, O.H. Akperov, 2012. Study of the Structure and Parameters of Grid of Hydrogenated Butadiene Nitrile Rubber cross-linked with Polymer Peroxides. Amer. J. of Pol. Sci. 2(5): 122-128 DOI: 10.5923/j.ajps.20120205.07 |
| [14] | Mammadov S.M., Hasanov V.Y., Garibov A.A. et.al. Radiasiya üsulu ilə vulkanizasiya edilmış rezin qarışığı. AZE. Patent I 20090198: IPC C 08L 9/00, C 08L 9/02 16.11.2009 |
| [15] | Mammadov S.M., Garibov A.A., Karimov M.K. et.al. Formalı və formasız texniki-rezin məmulatların hazırlanması üçün tərkib. AZE. Patent I 20120069: 31.07.2012 |
| [16] | Mammadov S.M., Garibov A.A., Salehov A.K. et.al. Butadien-nitril kauçukunun əsasında vulkanlaşdırılmalı rezin qarışığı. AZE. Patent I 20110094: 14.10.2011 |
| [17] | R.J. Woods, A.K. Pikayev. Applied radiation chemistry: radiation processing. J. Wiley, Science, 1994, 535 p. |
| [18] | Flory P.J., Rehner J.I., 1943, Statistical Mechanics of Cross‐Linked Polymer Networks I. Rubberlike Elasticity. J. Chem. Phys., Is. 11, pp 512-520 |
| [19] | O'Keefe, Jerome F., 2004. Identification of polymers by IR spectroscopy. Rubber World, |
| [20] | Roy Crompton. Determination of additives in polymers and Rubbers. Rapra Technology. 2007, 68 p. |
| [21] | D.I. Bower, W.F. Maddams. The vibrational spectroscopy of Polymers. Cambridge University Press. 1992, 337 p. |







 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML



