-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Polymer Science
p-ISSN: 2163-1344 e-ISSN: 2163-1352
2013; 3(4): 70-75
doi:10.5923/j.ajps.20130304.02
Poly(ε-caprolactone) Degradation Under Acidic and Alkaline Conditions
Aurelio Ramírez Hernández, Oscar Crisanto Contreras, Jorge Conde Acevedo, Leticia Guadalupe Navarro Moreno
Campus Tuxtepec, Circuit Central #200, Col. Park Industrial, University of Papaloapan, Tuxtepec, C.P. 68301, Oaxaca, México
Correspondence to: Aurelio Ramírez Hernández, Campus Tuxtepec, Circuit Central #200, Col. Park Industrial, University of Papaloapan, Tuxtepec, C.P. 68301, Oaxaca, México.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
In this paper, the chemical degradation of poly(ε-caprolactone) by hydrolysis was carried out. Poly(ε-caprolactone) was successfully synthesized via the ring-opening polymerization of ε-caprolactone at 155℃ with ammonium heptamolybdate. Poly(ε-caprolactone) was characterized by Fourier Transform Infrared Spectroscopy. The hydrolyses were carried out under acid and alkaline conditions using HCl, H2SO4, NaOH, and KOH. The temperature used in the hydrolyses ranged from 40℃ to 140℃, while the solution concentrations, time, and the mass of the samples during the chemical degradation were held constant. Under these degradation conditions a loss of weight of poly(ε-caprolactone) was observed when the temperature was increased. For example, for 30 minutes of degradation using sulfuric acid at temperatures of 120℃ and 140℃, the poly(ε-caprolactone) was degraded 25.00% and 36.31% respectively. The alkaline hydrolysis process is more rapid than acid hydrolysis. For example, after 30 minutes in NaOH at temperatures of 120℃ and 140℃, the poly(ε-caprolactone) was degraded 94.60% and 100%, respectively.
Keywords: Degradation, Biodegradable, Poly(ε-caprolactone), Acid and Alkaline Hydrolysis
Cite this paper: Aurelio Ramírez Hernández, Oscar Crisanto Contreras, Jorge Conde Acevedo, Leticia Guadalupe Navarro Moreno, Poly(ε-caprolactone) Degradation Under Acidic and Alkaline Conditions, American Journal of Polymer Science, Vol. 3 No. 4, 2013, pp. 70-75. doi: 10.5923/j.ajps.20130304.02.
Article Outline
1. Introduction
- Polymers are currently produced in large quantities in order to improve the quality of life for many people, and also polymers let contribute to the development of science and technology. But one major disadvantage of products elaborated with synthetic polymers is the negative effect on the environment when they are thrown out. For this reason, biodegradable polymers are being produced in laboratories [1-3], such is the case of poly(ε-caprolactone) (PCL), which is a semi-crystalline thermoplastic polyester[4-5]. PCL is synthesized by the ring opening polymerization (ROP) of ε-caprolactone (ε-CL), it is composed of five methylenes [(CH2)5] and an ester functional group [CO2] as the repeating unit. PCL and its derivatives are polymers that have been of great interest in the pharmaceutical industry as matrices for medical formulas, for this reason, PCL is used in therapeutic medicine due to its high permeability and low toxicity. Another one of the applications of PCL is in the production of degradable plastic bags. The ester functional group of PCL tends to be hydrolyzed and fragmented into short polymeric chains with low molecular weight. It has been reported that PCL is degraded by microorganisms, enzymes, and also chemical degradation methods (hydrolysis)[6-10]. In the case of the chemical methods, questions arise: Which is faster: the degradation of PCL in acidic or alkaline conditions? how can the degradation process be described?In this paper, an evaluation and comparison of the chemical methods for PCL degradation using acidic and alkaline hydrolysis is carried out.
2. Description of Experiment
2.1. Equipment
- - IKA RCT basic electric hot plate with magnetic stirrer.- Reflux water system.- Perkin-Elmer spectrum 100 FT-IR spectrometer.- Thermo Scientific vacuum pump.- 1H-NMR: Varían Gemini 2000 and Varian unity Plus 300.
2.2. Reactants
- - ε-Caprolactone 99% (Sigma Aldrich).- Ammonium heptamolybdate tetrahydrated (Riedel-de Haën).- Anhydrous methanol 99.85% (Baker).- Chloroform 99.97% (Golden Bell).- Hydrochloric acid 37.8% (Chemical Meyer).- Sulfuric acid 98.4% (Baker Analyzed).- Potassium hydroxide 86.03% (Chemical Meyer).- Sodium hydroxide 98.2% (Golden Bell).
2.3. Methods
2.3.1. PCL synthesis
- Sixty millimeters of ε-caprolactone, 0.3ml of water and 33mg of ammonium heptamolybdate were placed a 100ml around bottom flask, which was set up in a reflux system for 120 minutes at 155°C. The mixture was stirred at 250 rpm (see Figure 1).
 | Figure 1. PCL synthesis process |
2.3.2. PCL Degradation
- The mixture of 2.5g PCL and 30ml acid or alkaline solution was placed in a 50ml round bottom flask and set up to reflux for 30 minutes. The solution was stirred at 250rpm. The degradation temperature interval was 40ºC to 140°C. The percent of non-degraded PCL was determined using the equation:
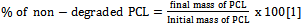
2.3.3. PCL Characterization
- The characterization of PCL and non-degraded PCL was by Fourier Transform Infrared Spectroscopy (FT-IR) and Nuclear Magnetic Resonance (NMR) spectroscopy.
3. Results and Discussion
3.1. PCL Synthesis
- PCL was synthetized from the polymerization of ε-caprolactone using ammonium heptamolybdate as a catalyst at 155ºC (see Figure 2).
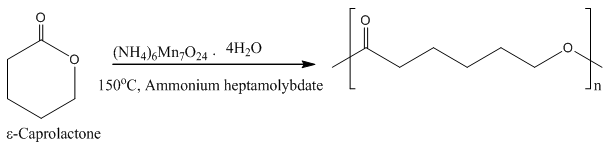 | Figure 2. Synthesis of poly(ε-caprolactone) (PCL) |
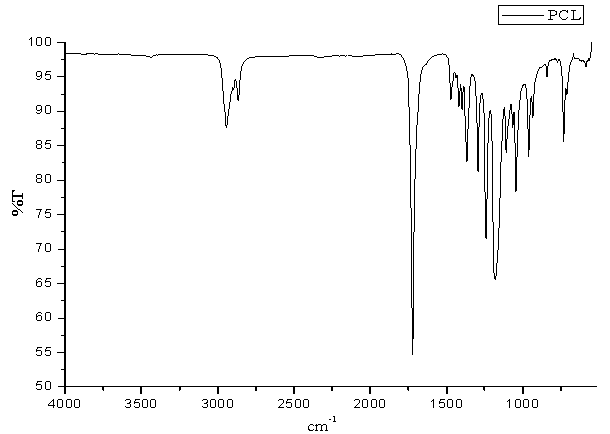 | Figure 3. FT-IR Spectrum of PCL |
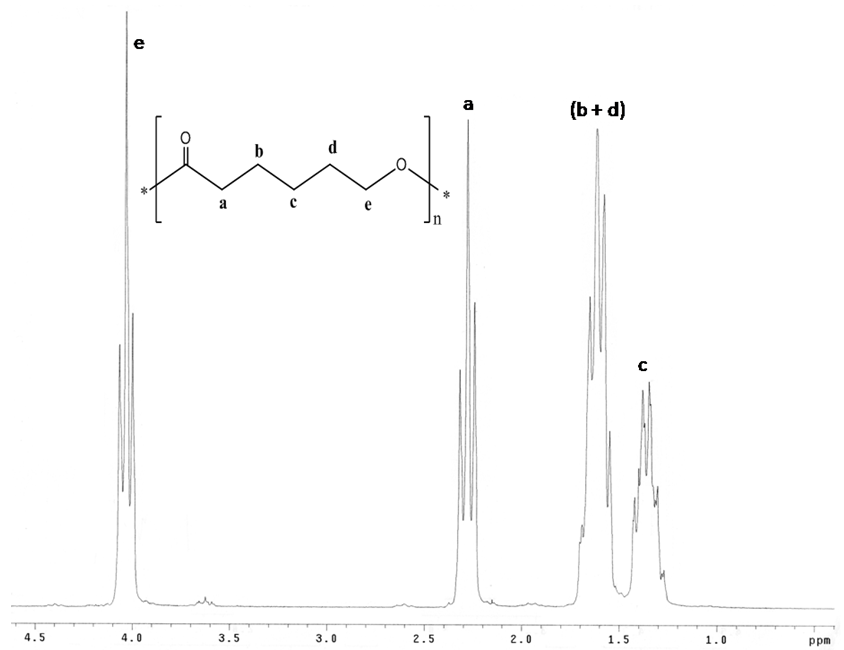 | Figure 4. Nuclear Magnetic Resonance spectrum (1HNMR) of PCL |
3.2. PCL Degradation
- In Table 1, we present the mass percentages of the PCL that were obtained by using equation[1] after the degradation processes, the solutions that were used for each degradation process are also specified in Table 1.
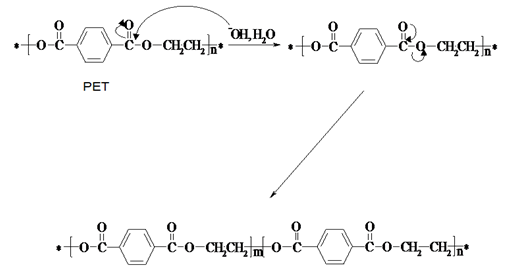 | Figure 5. Suggested alkaline degradation mechanism of poly(ethylene terephthalate), where “m” is less than “n”[11] |
 | Figure 6. Residue degradation of PCL |
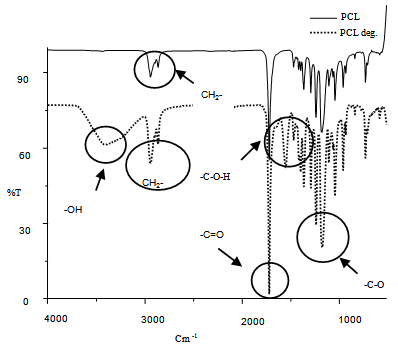 | Figure 7. The comparison FT-IR spectra of PCL and PCL non-degraded |
4. Conclusions
- The comparison reported in this work between the chemical degradation of PCL using acid and base solutions, allows us to conclude that when we used base solutions we obtained better degradation results than when we used acid solutions. This is due to the fact that the degradation process is faster and more efficient in alkaline media. In basic solutions the nucleophilic (OH)- and electrophilic (K+, Na+) entities react with the monomer of the repeating unit of the polymer more easily than when using acid [Cl-, (SO4)-2, H+], this reaction fractures the polymeric chain of the PCL and as a result the oligomers are obtained. The degradation type that PCL exhibits probably could be present in polymers that have similar structure. With regards to the basic solutions used in this work, the most effective solution for degradation was NaOH.
ACKNOWLEDGMENTS
- We are grateful to Jared J. Gerschler, Leticia Medina Saldaña and the University of Papaloapan for the support required to accomplish this research work.
References
| [1] | Martínez, G., MatosM., Sabino, M.,Urbina de Navarro, C.,Barrios,C., Taddei, Sajo,A., Arnal,M., Müller, A., Study of a binary mixture biodegradable: polycaprolactone /chitin”, Rev. Iberoamericana of polímers, 3(9): 313-321, 2008. |
| [2] | Martínez G, Urbina de Navarro C, Barrios C, Matos M, Sabino M, Müller A.J, Taddei A, Castelli C. Crystallization, morphology and enzymatic degradation of polyhydroxybutyrate/polycaprolacton, J. Macromolecular Chemistry and Physics, 208, 924-937, 2007. |
| [3] | Chang yong, C., Young, Tai-Hyoung, K., Mi-Kyeong, J., Chong Su, C., Jae-Woon, N., Preparation and Characterizations of Poly(ethylene glycol)-Poly (ε-caprolactone) Block Copolymer Nanoparticles, Bull. Korean Chem. Soc. 2005, 4(26), 523-528, 2005. |
| [4] | Báez García, E., Ramírez Hernandez, A., Marcos A., Synthesis, characterization, and degradation of poly(ethylene-b-ε-caprolactone) diblock copolymer Polym. Adv. Technol. 21(1),55-64, 2010. |
| [5] | Jenkins, M., Harrison, K., The effect of molecular weight on the crystallization kinetics of polycaprolactone, Polym. Adv. Technol. 17(6), 474-478, 2006. |
| [6] | Lian song, W., Zhiping. Z., Hechun, C., Shenglan Z., Chengdong. X., Preparation and characterization of biodegradable thermoplastic Elastomers (PLCA/PLGA blends), J. Polym. Res. 2010,17(1), 77-82, 2010. |
| [7] | Shao bing, Z., Z., Xianmo, D., Hua, Y., Biodegradable poly(e-caprolactone) poly(ethylene glycol) block copolymers: characterization and their use as drugcarriers for a controlled delivery system, Bioamaterials, 24, 3563-3570, 2003. |
| [8] | Abe, H., Takahashi, N., Ju Kim, K., Mochizuki, M., Doi Y., Thermal Degradation Processes of End-Capped Poly (l-lactide)s in the Presence and Absence of Residual Zinc Catalyst, Biomacromolecules, 5(4), 1480-1488, 2004. |
| [9] | Báez, J.E., Poli(ε-caprolactone), degradable polymer: synthesis triisopropoxide aluminum Al (OiPr)3 as initiator, Education chemistry, 17(4), 458-463, 2006. |
| [10] | Mukerjee, A., Sinha, V.R, Pruthi, V., Preparation and Characterization of Poly-ε-caprolactone Particles for Controlled Insulin delivery, Journal of biomedical and Pharmaceutical engineering, 1(1), 40-44, 2007. |
| [11] | A. Ramírez. 2002. Study depolymerization of poly (ethylene terephthalate). [online]. Available: http://quimica.ugto.mx... 2007 |
| [12] | Rahman, M., Alfaro., M., Degradation of polyester geotextiles in alkaline solutions under applied loading. Dep. University of Manitoba, Winnipeg, Canada. Géo Québec. 2004; 33-39. |
| [13] | Muralidharak, S., Sreenivasan, S., Thermal degradation and burning behavior of cotton polyester and polyester/cotton blended upholstery, World Applied Sciences Journal, 9 (11), 1272-1279, 2010. |
| [14] | Sanz, B. C., Salvador, M. D., Segovia López, F., Borrás, V., Pérez, A.T., Reinforced Plastics chemical degradation in basic media, VIII National Congress of Mechanical Properties of Solids. Gandia, 341-347, 2002. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML