-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Polymer Science
p-ISSN: 2163-1344 e-ISSN: 2163-1352
2013; 3(4): 63-69
doi:10.5923/j.ajps.20130304.01
Studies on the Effects of Various Flame Retardants on Polypropylene
Balaram Pani1, Sidhharth Sirohi1, Dhirendra Singh2
1Bhaskaracharya College of Applied Sciences, University of Delhi, Sec-2, Phase-1, Dwarka, New Delhi, 110 075, India
2Indian Institute of Technology, Delhi-110 016, India
Correspondence to: Balaram Pani, Bhaskaracharya College of Applied Sciences, University of Delhi, Sec-2, Phase-1, Dwarka, New Delhi, 110 075, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Polypropylene is a linear saturated hydrocarbon polymer. In this article, the impact of a typical fire retardant mixture of Ammonium Polyphosphate(APP) and Pentaerythritol(PER) on the fire retardant characteristics of polypropylene(PP) has been studied. The various impact of the fire retardant mixtures (APP-PER) was studied at different proportions of 3:1 and 4:1 on the PP. To observe the effect of the composition of mixtures of both (3:1 & 4:1) on PP was studied in the different mixing compositions (15%, 30% & 40%). In this experiment, the flame retardant(FR) was operated by intumescence method. The effect of various flame retardants on PP were studied by preparing a ternary mixtures of PP-APP-PER on Brabender mixture at 180C for 10 min at a speed of 80rpm. The mixture was hot pressed at 180C under10MPa pressure for 12 min to prepare sheets of suitable thickness for analysis. The prepared sheets were subjected to the laboratory test such as, Limiting Oxygen Index(LOI) and Thermogravimetric Analysis(TGA) to find out the burning behavior and changed in the weight of a specimen respectively. The flammability test was carried out to study the flame retardancy behavior of the specimen. The degradation of PP is influenced with the increase content of phosphorous in the APP-PER mixture. LOI test indicates an improvement in flame retardancy of PP due to presence of high phosphorous content in 4:1 mixture.
Keywords: Flame Retardants, Polypropylene, Ammonium Polyphosphate, Pentaerythritol, Limiting Oxygen Index
Cite this paper: Balaram Pani, Sidhharth Sirohi, Dhirendra Singh, Studies on the Effects of Various Flame Retardants on Polypropylene, American Journal of Polymer Science, Vol. 3 No. 4, 2013, pp. 63-69. doi: 10.5923/j.ajps.20130304.01.
Article Outline
1. Introduction
- Polypropylene has a wide range of application in the field of textiles. The morphological structure of polypropylene is rather complex and at least four different types of spherullite have been observed. The properties of the polymer depend on the size and types of crystal structure formed. These properties are generally influenced by the ratio of the relative rates of nucleation to the crystal growth. The ratio of these two rates can be controlled by varying the rates of cooling and by the incorporation of nucleating agents. In general, the smaller the crystal structure, the greater is the transparency and flex resistance, lesser the rigidity and heat resistance. Polypropylene is widely used in a large variety of common polymers as thermoplastic material because of its excellent mechanical properties, low density, good thermal and chemical resistance and easy processing. The polypropylene has limited use on electric and electronic materials because of their easy flammability with low limiting oxygen index which are the order of 18%[1]. In order to improve the flame retardancy of polypropylene, reinforcing agents and flame retardants are added to PP. Commercial polymers are usually about 90%-95% isotactic. The isotactic polypropylene has low density and high softening point. Polypropylene appears to be free from environmental stress cracking resistance. The only exception seems to be with concentrated sulfuric acid, chromic acid and aqua regia. It is more susceptible to oxidation at elevated temperatures and it has higher brittle point. The importance of flame retarding thermoplastics is to increase the resistance of a material to ignition and once ignited, to reduce the rate of flame spread. The type of flame retardant and the quantity needed to meet specific objectives depend on the specific polymer. Various halogen or phosphorus[2] containing flame retardants such as metal hydroxides and oxides[3], nano-composites[4] and intumescent[5] systems are usually synergistically applied in PP composites to improve their flame retardant efficiency[6].Additive as well as reactive flame retardants are available commercially. The addition of large quantities of flame retardants may severely degrade the properties of thermoplastic and may also present processing problems. Flame retardants (FR) that plasticize the polymer reduce thermal properties such as heat distortion temperature whereas non melting solid additives may severely degrade impact properties. To enhance the FR efficiency of intumescent flame retardants (IFRs) many synergists have been introduced into the system, including sepiolate[7], vermiculate[8], fumed silica[9], iron powder[10], metal oxides[11], nickel phosphates[12], zeolites[13] andmentmorillnate[14].The adverse effect of flame retardants on the environment may be reduced by using various halogen free flame retardants and intumescent flame retardants. Polypropylene is of great importance in use because of little smoke and low toxicity during burning[15-19]. These retardants also produce very low toxic smog and anti dripping. In this study, care has been taken to find out the effects of various flame retardants on PP as well as to meet the environmental problems. The objectives of this study are to study the impact of a typical fire retardant mixture of Ammonium Polyphosphate and Pentaerythritol (APP-PER) on the fire retardant characteristics of Polypropylene (PP). The various impact of the fire retardant mixtures (APP-PER) at different proportions (here 3:1 and 4:1) on the PP has also been studied. The composition of mixtures of both (3:1 and 4:1) fire retardant additive mixtures and PP also studied in different mixing compositions (15%, 30% and 40%) to observe the impact. The study is also meant for comparing the oxygen indices of these mixtures measured before and after addition of additives. The relative effect of fire retardant additive is being determined by adding polypropylene to it. In this process the increasing or decreasing rate of thermal oxidation value of PP was observed. A comparative study of the efficiency of fire retardant additives is carried out by using different mixture compositions and addition proportions of fire retardants so that an overview of the trend on may be made.
2. Experimental
- Flame retardants may function by intumescence[20], polymerization[21], melt dripping[22], flame poisoning[23], charring[24, 25], cooling[26] and/or by forming protective coating[27]. In this experiment, the FR was operated by intumescence method.
3. Materials
- isotactic pp shows higher stability at higher temperature up to 100℃ and higher stress cracking resistance. Because of its tertiary carbon atoms, pp has a lower oxidative stability. PP is one of the lightest thermoplastics because of its low density (0.9 - 0.91). A large scale production of pp uses co ordination polymerization with Ziegler-Natta catalyst. The structure of PP is given in Fig. 1.
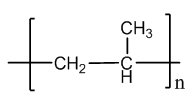 | Figure 1. Structure of Poly-Propylene |
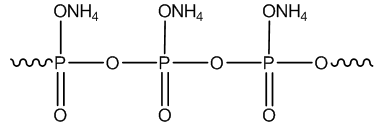 | Figure 2. Structure of Ammonium Polyphosphate |
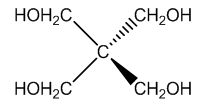 | Figure 3. Structure of Pentaerythritol |
4. Sample Preparation
- The intumescent flame retardants consist of APP, PER, and PP. The ratio of APP to PER was fixed at 3:1 & 4:1 and loading of these mixtures was kept at 15, 30 and 40 wt (%) to the PP in the IFR system respectively. All composites were prepared in a Brabender mixture at 180C for 10 min at a speed of 80 rpm. After mixing, the samples were hot pressed at 180C under 10 MPa pressure for 12 min into sheets of suitable thickness for analysis.
5. Methodology
5.1. Determination of Limiting Oxygen Index (LOI)
- A complete assessment of the oxygen index was conducted using top surface ignition. A small test specimen was supported vertically in a mixture of oxygen and nitrogen flowing upwards through a transparent chimney. The upper end of specimen was ignited and the subsequent burning behavior of the specimen was observed to compare the period for which burning continues or the length of specimen was burnt with specified limits for each burning. By testing a series of test specimens burning in different oxygen concentration, the minimum oxygen index (the minimum concentration of oxygen) was determined by this method expressed in terms of volume percent in a mixture of oxygen and nitrogen which just support the flaming combustion of material initially 23±2oC under the conditions of test method. As per this procedure, LOI data of all samples were obtained at room temperature on an oxygen index instrument (Oxygen Index tester- S.A Associates, Delhi). Based on ASTM D2863-77 standard, the dimensions of all samples taken in this experiment were 130 x 6.5 x 3 mm3[28].
5.2. Thermogravimeteric Analysis
- Thermo-gravimetric analysis (TGA) is a technique in which the weight change of a specimen is monitored with progressive heating. The components of the sample decomposed at different temperatures. This leads to a series of weight loss that allow the components to be quantitatively measured. A typical high performance apparatus consists of an analytical balance supporting a platinum crucible fixed in an electric furnace was used for the experimental work.TGA is very useful in characterizing polymers containing different levels of additives by measuring the degree of weight loss. The thermal stability of a polymer can be obtained through a kinetic analysis of the decomposition profile. TGA was performed on a TGA-1500 (Rheometric Scientific) at a heating rate of 10℃ min-1. Sample weight was kept within 5 to 10mg. The sample was examined under pure nitrogen condition at a flowing rate of 50ml min-1 at temperatures ranging from 50 to 700℃.
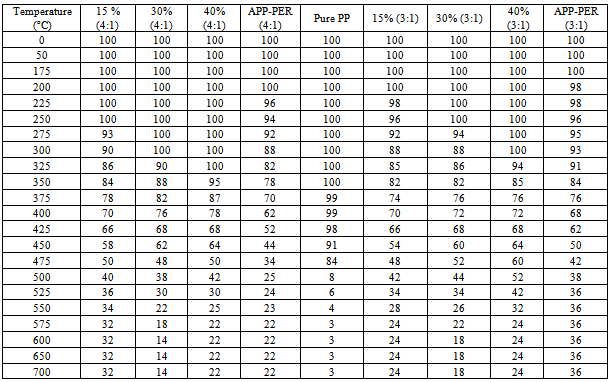
6. Results & Discussion
- The results obtained from the experimental work of limiting oxygen index and thermo gravimetric analysis of the samples are given in the Table No.1 and 2 respectively.
|
|
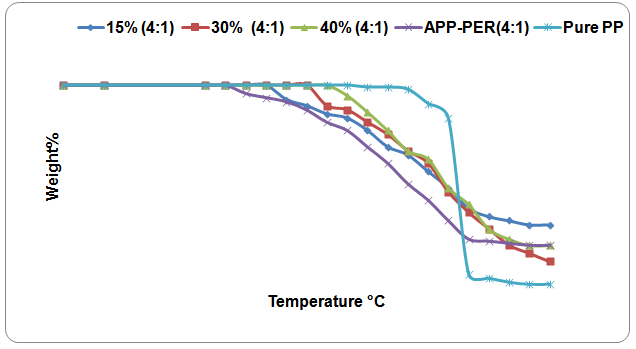 | Figure 4. TGA curves of PP-APP-PER (APP-PER 4:1 w/w) mixture with PP at different Proportions (15%, 30%&40%) Temperatures |
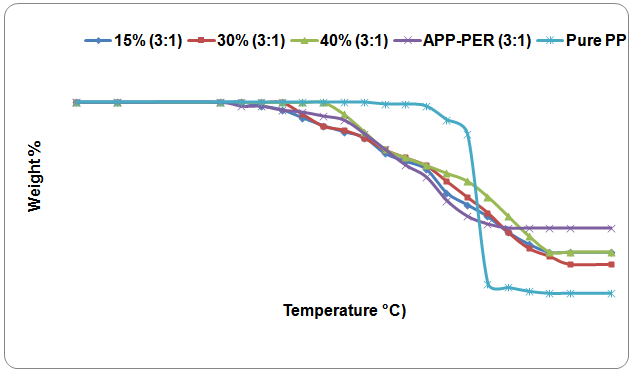 | Figure 5. TGA curves of PP-APP-PER(APP-PER 3:1 w/w) mixture with PP at different Proportions (15%,30%&40%) Temperatures |
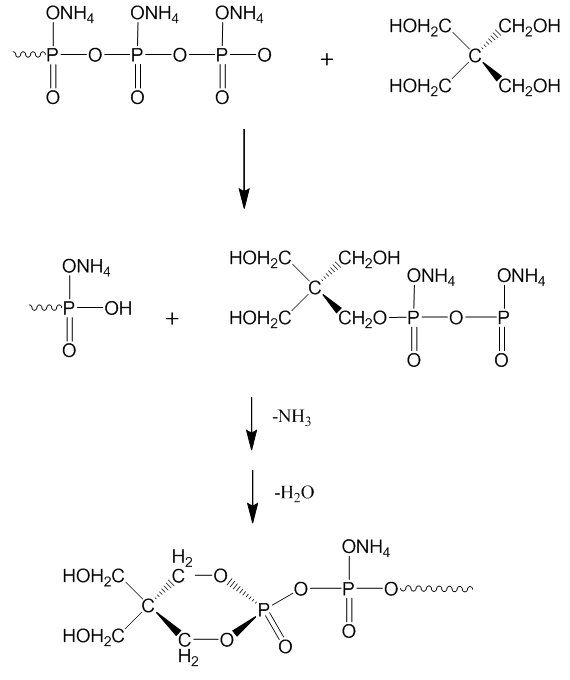 | Figure 6. Chemical reaction of APP and PER |
7. Conclusions
- the thermal degradation of Polypropylene mixed with fire retardant APP-PER (4:1) has lower value than that of (PP-APP-PER) mixture(3:1). It may be concluded that the fire retardancy property of polymer increases with the increasing content of phosphorous in FR. The composition of volatile products formed by degradation of PP in presence of the additives(both 3:1 and 4:1) has little impact on the properties of the polymer. The effective flame retardancy of PP is more because of the higher phosphorous content (4:1) in the fire retardant mixture(APP-PER). It was also confirmed from the result obtained from LOI test.As the loading percentage of additives increases, the effective flame retardancy of PP also increases but less significantly. The fire retardant concentration has been kept at optimum level. The loading percentage has not been increased beyond 40% assuming that the physical as well as chemical properties of PP will be affected adversely.
ACKNOWLEDGEMENTS
- We acknowledge the Bhaskaracharya College of Applied Sciences, University of Delhi, Delhi and Jawahar Lal Nehru University, New Delhi for supporting us to carry out the laboratory work in the institution laboratory.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML