-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Polymer Science
p-ISSN: 2163-1344 e-ISSN: 2163-1352
2013; 3(3): 35-45
doi:10.5923/j.ajps.20130303.01
Preparation, Compatibility Studies and Evaluation of Polymer Mixture of Decyl Acrylate and its Copolymer with Styrene as Lubricating Oil Additives
Pranab Ghosh, Debabrata Nandi, Tapan Das
Natural Product and Polymer Chemistry Laboratory Department of Chemistry, University of North Bengal, Darjeeling, 734013, India
Correspondence to: Pranab Ghosh, Natural Product and Polymer Chemistry Laboratory Department of Chemistry, University of North Bengal, Darjeeling, 734013, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Polydecyl acrylate (PDA) and copolymer of decyl acrylate (DA) with styrene and these polymeric samples were prepared, characterized and evaluated as additives for lubricating oil. Viscometric studies have been conducted on the binary solutions (polymer-solvent) of polydecyl acrylate (PDA), copolymer of DA as well as on their ternary solutions (polymer-polymer-solvent) having different concentrations of each polymer at 30°C in chloroform and toluene. The estimation of the compatibility degree of the above polymer pairs has been made by means of five criteria. Results show that in most of the cases polymer blends are compatible, except 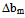 data. This is probably due to non-ideal behavior (hydrodynamic interaction) of polymers in ternary solutions. Data obtained in the solvents are not similar implying that polymer-polymer interactions and polymer-solvent interactions are both important for long chain polymers. Two solvents were chosen based on their ability to dissolve the polymers and, due to their differences in both structure and properties. The evaluation data indicates that compatible polymer mixtures act as much better viscosity index improvers (VII) and pour point depressants (PPD) than both the homopolymer and copolymer. With increasing concentration of the copolymer in the blend, there is gradual increase in the additive performance.
data. This is probably due to non-ideal behavior (hydrodynamic interaction) of polymers in ternary solutions. Data obtained in the solvents are not similar implying that polymer-polymer interactions and polymer-solvent interactions are both important for long chain polymers. Two solvents were chosen based on their ability to dissolve the polymers and, due to their differences in both structure and properties. The evaluation data indicates that compatible polymer mixtures act as much better viscosity index improvers (VII) and pour point depressants (PPD) than both the homopolymer and copolymer. With increasing concentration of the copolymer in the blend, there is gradual increase in the additive performance.
Keywords: Viscometric Studies, Ternary Solutions, Compatibility
Cite this paper: Pranab Ghosh, Debabrata Nandi, Tapan Das, Preparation, Compatibility Studies and Evaluation of Polymer Mixture of Decyl Acrylate and its Copolymer with Styrene as Lubricating Oil Additives, American Journal of Polymer Science, Vol. 3 No. 3, 2013, pp. 35-45. doi: 10.5923/j.ajps.20130303.01.
Article Outline
1. Introduction
- Rheological properties of mineral lubricating oils, particularly their viscosity index and pour point, are being considerably improved by the addition of certain polymers in low concentrations having controlled and defined structural characteristics[1-2]. The most efficient and most commonly used polymers as viscosity index improvers and pour point depressants for lubricating oils are polymers of statistical olefins, long chain acrylates and methacrylates and their copolymers with different olefins (usually styrene, maleic anhydride, 1 – decene etc.)[1,3]. Recently because of their field application, exploration of polymeric additive mixtures as viscosity modifiers and pour point depressant for lubricating oils have intensified since they have shown some complimentary and even synergetic effects in solvents[1,3]. However, due to physico-chemical differences, many of the polymers are incompatible; they generate separate phases readily, particularly in solutions. Polymer-polymer compatibility has been extensively studied by several techniques, such as differential scanning calorimetry, dynamic mechanical measurements, neutron scattering, inverse gas chromatography, electron microscopy, light scattering and others, most of which are experimentally demanding and time-consuming techniques. And, for these reasons, an alternative, simple and reliable method to analyze polymer-polymer miscibility in solution is the viscometric technique.When two different polymers are dissolved in a solvent, they interact (polymer–polymer interaction) with each other. These interactions are mainly of two types, hydrodynamic and thermodynamic. These two interactions, together, influence the compatibility (attraction or repulsion) of the polymer mixture. If the polymers attract each other, called as compatible, the effective hydrodynamic volume of the polymer blend is higher than the sum of the two individual polymers in solution. On the contrary, when there is repulsion between the polymers, called as incompatible, the effective hydrodynamic volume of the polymer blend is lower than the sum of the two individual polymers in solution. Again with variation of percentage of each polymer, the compatibility of the polymer mixtures varies, for the interaction between them depends on the individual hydrodynamic volume of the polymers and on the polymer–solvent interaction[4-6]. Together with polymer–polymer interaction, polymer-solvent interaction also plays an important role on the compatibility of polymer mixture in ternary system[4-6]. When two different polymers are dissolved in a common solvent, the hydrodynamic volume and configuration of the polymers get affected greatly. Hence, the compatibility parameter values of the polymer mixtures will be different in different solvents. According to literature, the compatible or miscible polymer mixtures show better performance as VII and PPD than the individual homo polymers and even copolymers [7-9]. Most of the base oils contain some parafinic wax. In cold temperature the wax crystallizes to form a rigid structure which, with their small pockets, traps oil[10]. With sufficient formation of these crystal structures the oil will no longer be capable to flow. To overcome this problem certain high molecular weight polymers are used with base oils as PPD. The polymer inhibits the formation of wax crystals by adsorption followed by cocrystallyzation which redirect the wax crystal structures to smaller size and thus increases the solubility of crystals in base oil[11]. The effectiveness of the polymers as PPD depends on their chemical composition and structural characteristics[12-14]. A polymer with higher hydrodynamic volume, in base oil, performs better as PPD. Viscosity index improvers (VII) are those which modify the rate of change of viscosity of base oil with temperature. Certain high molecular weight polymers are used as VII to increase considerably the base oil viscosity of at high temperature but at low temperature they increase the base oil viscosity a little. With rising temperature the viscosity of base oil decreases but its solubility increases. At low temperature the polymer molecules remain as a tight coil in base oil but with increasing temperature the hydrodynamic volume of polymer molecules increases which counterbalances the normal reduction of viscosity of base oil with temperature[15]. When the concentration of a polymer increases in base oil, total volume of the polymer micelles also increases which imparts a higher viscosity index[16]. Similarly, a higher concentration of a compatible polymer blend will contributes a higher viscosity index to the base oil than a lower concentration of the same polymer blend.The compatibility and evaluation of PDA with its copolymer with styrene has not been thoroughly investigated earlier. The present investigation, thus, comprises the preparation of PDA and copolymer of PDA with styrene followed by a comparative study on the viscosity behavior of PDA, its copolymer with styrene and mixtures of PDA with its copolymer in two different solvents, toluene and chloroform by following five criteria: (1) viscosity interaction parameter
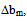 which is used from the equations developed by both Krigbaum and Wall, and their modified forms by Williamson and Wright[17-19]; (2) viscosity interaction parameter
which is used from the equations developed by both Krigbaum and Wall, and their modified forms by Williamson and Wright[17-19]; (2) viscosity interaction parameter 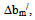 as was first introduced by Campos et al[20]; (3) viscosity interaction parameter α, as was developed by Sun et al.[20]; (4) viscosity difference parameter
as was first introduced by Campos et al[20]; (3) viscosity interaction parameter α, as was developed by Sun et al.[20]; (4) viscosity difference parameter 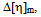 treated as an excess property for compatibility of polymer mixtures and (5) change of slope of the curve in the plot of reduced viscosity against concentration in ternary system as was proposed by Yang Haiyang et al.[4, 22]. Homo and copolymers of PDA have been characterized by Thermo gravimetric analysis (TGA), FT-IR and -NMR spectra. Then we have evaluated these polymers and their polymer blends as PPD and VII in two different base stocks from same source[23].
treated as an excess property for compatibility of polymer mixtures and (5) change of slope of the curve in the plot of reduced viscosity against concentration in ternary system as was proposed by Yang Haiyang et al.[4, 22]. Homo and copolymers of PDA have been characterized by Thermo gravimetric analysis (TGA), FT-IR and -NMR spectra. Then we have evaluated these polymers and their polymer blends as PPD and VII in two different base stocks from same source[23].2. Theory
- Many groups of workers[18-22, 24] have investigated the miscibility of polymer blends by carrying out viscosity measurements of the corresponding ternary(polymer-polymer-solvent) systems. These methods rely on different assumptions. We choose some of these methods to characterize our polymers and polymer blends.
2.1. According to the Huggins’ Equation[17], the Value of Intrinsic Viscosity Changes with the Concentration C of a Single Solute Solution (Binary System) as
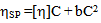 | (1) |
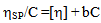 | (2) |
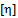 is the intrinsic viscosity and b is the polymer–polymer interactions at finite concentrations related to the Huggins coefficient kH, by the equation:
is the intrinsic viscosity and b is the polymer–polymer interactions at finite concentrations related to the Huggins coefficient kH, by the equation: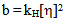 | (3) |
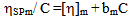 | (4) |
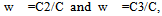 Where w2 and w3 are the weight fractions of polymer 2 and polymer 3 respectively. Theoretically intrinsic viscosity of a ternary solution, m could be deduced as:
Where w2 and w3 are the weight fractions of polymer 2 and polymer 3 respectively. Theoretically intrinsic viscosity of a ternary solution, m could be deduced as: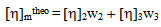 | (5) |
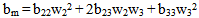 | (6) |
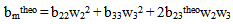 | (7) |
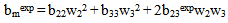 | (8) |
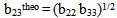 | (9) |
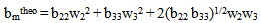 | (10) |
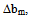 which determines the intermolecular interaction between polymer 2 and polymer 3 in ternary solutions, can be evaluated as:
which determines the intermolecular interaction between polymer 2 and polymer 3 in ternary solutions, can be evaluated as: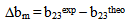 | (11) |
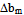 as:
as: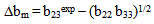 | (12) |
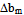 for compatibility in polymer mixtures in ternary solutions is based on the comparison between experimental (b23exp) and theoretical or ideal (b23theo) values of b23[17]. The values of b23exp > b23theo or
for compatibility in polymer mixtures in ternary solutions is based on the comparison between experimental (b23exp) and theoretical or ideal (b23theo) values of b23[17]. The values of b23exp > b23theo or 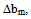 > 0 indicates that polymers are compatible in polymer mixtures or attractive molecular interaction exists between polymers and values of b23exp < b23theo or
> 0 indicates that polymers are compatible in polymer mixtures or attractive molecular interaction exists between polymers and values of b23exp < b23theo or 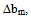 < 0 indicates incompatibility between the polymers or repulsive molecular interaction.
< 0 indicates incompatibility between the polymers or repulsive molecular interaction.2.2. The Second Compatibility Criterion, 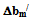 is the Difference between bmexp and a New Viscometric Interaction Parameter, bmtheo/ such as
is the Difference between bmexp and a New Viscometric Interaction Parameter, bmtheo/ such as
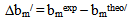 | (13) |
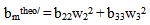 | (14) |
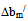 > 0, the polymers are compatible and when bmexp < bmtheo/ or
> 0, the polymers are compatible and when bmexp < bmtheo/ or 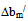 < 0, the polymers are incompatible.
< 0, the polymers are incompatible.2.3. The Third Compatibility Parameter, α, Proposed by Sun et al.[21] for the Determination of Polymer Miscibility is as Follows
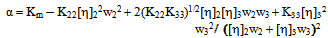 | (15) |
2.4. The forth Compatibility Parameter, 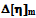 is Based on the Difference between the Experimental and Theoretical or Ideal Values of
is Based on the Difference between the Experimental and Theoretical or Ideal Values of 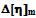 as
as
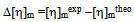 | (16) |
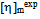 is determined from the intercept of plots for Eq. (4) and
is determined from the intercept of plots for Eq. (4) and 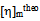 is calculated with Eq. (5) using the data from the binary systems. The values of
is calculated with Eq. (5) using the data from the binary systems. The values of 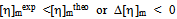 indicate compatibility between polymers whereas
indicate compatibility between polymers whereas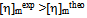 or
or 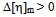 indicates incompatibility between the polymers.The fifth compatibility criterion, change of the slope of the curve in the plot of reduced viscosity against concentration in ternary system was proposed by Yang Haiyang et al.[4,22].They proposed that as in ternary system there exists either attraction or repulsion between polymers which changes effective hydrodynamic volume of the molecules and thus influences on the viscosity of ternary solutions. If attraction exists between polymers (compatible), then mutual hydrodynamic volume of polymers increases which will lead to the positive deviation of curve. On the other hand, repulsion between the polymers (incompatible) leads to the decrease of their mutual hydrodynamic volume which is indicated by decrease in slope of the curve. So the change of slope in the plot of
indicates incompatibility between the polymers.The fifth compatibility criterion, change of the slope of the curve in the plot of reduced viscosity against concentration in ternary system was proposed by Yang Haiyang et al.[4,22].They proposed that as in ternary system there exists either attraction or repulsion between polymers which changes effective hydrodynamic volume of the molecules and thus influences on the viscosity of ternary solutions. If attraction exists between polymers (compatible), then mutual hydrodynamic volume of polymers increases which will lead to the positive deviation of curve. On the other hand, repulsion between the polymers (incompatible) leads to the decrease of their mutual hydrodynamic volume which is indicated by decrease in slope of the curve. So the change of slope in the plot of 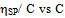 in a ternary system can be used as a criterion to determine polymer-polymer compatibility.
in a ternary system can be used as a criterion to determine polymer-polymer compatibility.3. Experimental
3.1. Materials Used
- Acrylic acid (GC Purity 99%), procured from Thomas Baker, India, Decyl alcohol (GC Purity 98%), obtained from S. D Fine Chemicals Ltd, India, styrene obtained from Merck Products, Germany and Hydroquinone procured from S. D Fine Chemicals Ltd, India, were used. Benzoyl peroxide (GC Purity 98%), procured from Loba Chemicals Pvt. Ltd., India , was purified by crystallization from methanol –chloroform mixture and was used. Toluene (GC Purity 99.5 %), obtained from Merck, India, was used as a solvent.
3.2. Esterification of Acrylic Acid with Decyl Alcohol
- Decyl acrylate was prepared by reacting methacrylic acid with decyl alcohol (1.1:1 molar ratio). The reaction was carried out in a resin kettle in the presence of catalytic amount of concentrated sulphuric acid, 0.25% hydroquinone (with respect to the monomer) as polymerization inhibitor for acrylic acid, and toluene as a solvent under a slow stream of deoxygenated nitrogen. The reactants, which were mixed with toluene, were heated gradually from room temperature to 403 K using a well-controlled thermostat. The progress of the reaction was followed by monitoring the amount of liberated water from the reaction mixture to give the ester, decyl acrylate.
3.3. Purification of Prepared Ester
- The prepared ester was purified according to the following procedure: a suitable amount of charcoal was added to the ester, allowed to reflux for 3 h, and then filtered off. The filtrate was washed with 0.5N sodium hydroxide in a separating funnel and then shaken well. The entire process was repeated several times to ensure complete removal of unreacted acid. The purified ester was then washed several times with distil water to remove any traces of sodium hydroxide, the ester was then left over night on calcium chloride and was then removed by distillation under reduced pressure and was used in the polymerization process.
3.4. Preparation of Homo Polymer of DA and its Copolymer with Styrene and their Purification
- The polymerization was carried out in a four necked round bottom flask equipped with a stirrer, condenser, thermometer, an inlet for the introduction of nitrogen and a dropping funnel through which to add styrene drop wise. In the flask was placed desired mass of DA and initiator (BZP) followed by the desired mass of styrene was added drop wise for 2 h in the presence of toluene as solvent. The reaction temperature was maintained at 353 K for 6 h. At the end of the reaction time, the reaction mixture was poured into methanol with stirring to terminate the polymerization and precipitate the polymer. The polymer was further purified by repeated precipitation of its hexane solution by methanol followed by drying under vacuum at 313 K. A homo polymer of DA was similarly prepared and purified under the same conditions for use in reference experiments.
3.5. Spectroscopic Measurements
- IR spectra were recorded on a Shimudzu FT-IR 8300 spectrometer using 0.1 mm KBr cells and the spectra were recorded at room temperature within the wave number range 400 to 4000 cm-1. NMR spectra were recorded in a Brucker Avance 300 MHz FT-NMR spectrometer using 5 mm BBO probe. CDCL3 was used as the solvent and TMS as reference material.
3.6. Viscometric Measurements
- Viscosities were determined at 313K in chloroform and toluene, using an Ubbelohde OB viscometer placed in a thermostatically controlled bath. The temperature was measured close to the capillary by a thermometer with an accuracy of 0.01K. Experimental determination was carried out by counting time flow at least six different concentration of the sample solutions. The time flow of the solution was manually determined by using a chronometer. In a single measurement the lowest value of solution concentration was chosen for the calculation. The viscometer was calibrated frequently with distilled water. The viscosity results were checked against viscosity of known solutions and uncertainty was found to be nearly 0.17 %. Precautions regarding prevention of evaporation of solvent were taken in all the cases.All the binary solutions were prepared by dissolving a measured weight of the polymer in Chloroform and toluene and diluting to a measured volume. All the ternary solutions were prepared by dissolving a measured weight of the polymers with DA : copolymer of DA with styrene weight ratio of 3:1, 1:1 and 1:3 in chloroform and toluene and diluting to a measured volume.
3.7. Evaluation of Prepared Additive as Pour Point Depressants (PPDs) in Base Oils
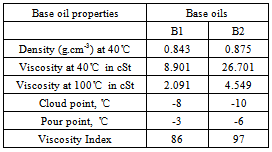
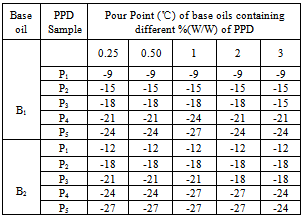
- The prepared additives were evaluated as pour point depressant using two different base oils collected from same source, (Table 5) through the pour point test according to the ASTM–D-97 method using WIL-471 cloud and pour point test apparatus model 3 (India). The effect of additive concentration was investigated by using different doping concentration for individual polymers (binary) and polymer blends (ternary). All the ternary blend solutions were prepared by dissolving a measured weight of the polymers with a DA : copolymer of DA with styrene weight ratio of 3:1, 1:1 and 1:3 in base oils. The experimental data were noted by taking an average of three experimental results under identical conditions.
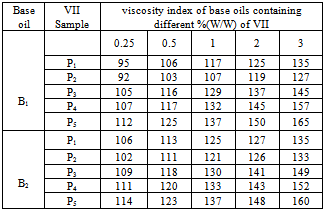
3.8. Evaluation of Prepared Additive as Viscosity Index Improvers (VIIs) in Base Oils
- Different binary and ternary blends were prepared by using two different types of base oils. Viscosities and the viscosity index (VI) of these oils were calculated according to ASTM D2270. Different weight percentages of concentration from 0.25 to 3.0 were used to study the effect of concentration on VI of the additive-doped lube oil. The experiment was done for both binary and ternary blends. In this respect, the kinematic viscosity of the oil containing different concentrations of the prepared polymers, copolymers and polymer blends ware determined at 313 K and 373 K.The experimental data were noted by taking an average of three experimental results under identical conditions.
4. Results and Discussion
- IR spectra of the homo polymer (Fig. 1) showed a peak at 1732 cm-1 due to the ester carbonyl group stretching vibration. The broad peak ranging from (1261 to 900 ) cm-1 appeared owing to the ester C-O stretching vibration along with the absorption bands at 977 and 711 cm-1 were due to the bending of C-H bond and from ( 3100 to 2900 ) cm-1 due to the stretching vibrations.The existence of the copolymer was confirmed by FT-IR (Fig. 2) and NMR analysis. The peak at 1732 cm-1 due to the presence of ester carbonyl group stretching vibration and the absorption bands at 758 and 702 were due to C-H bond of the phenyl group of styrene.In the 1H NMR spectra (Fig. 3) of the copolymer, a broad multiplet centered at 8.07 ppm indicated the presence of aromatic protons of phenyl group. A broad singlet at 4.06 ppm was due to the proton of the –OCH2 group. The absence of singlet between 5 and 6 ppm indicated the absence of vinylic protons in the copolymer.The extent of incorporation of styrene in the polymer chain was determined through a comparison of area of –OCH2 group at 4.06 ppm in the area of signal due to phenyl protons at 8.07 ppm based on earlier reports as well as on the basis of our earlier paper[23].The proton decoupled13 C NMR spectrum (Figure 4) of the above sample of copolymer was in complete agreement with the original structure-I.
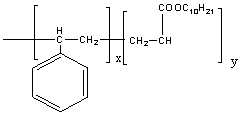 | Structure 1. Copolymer of Decyl acrylate with styrene |
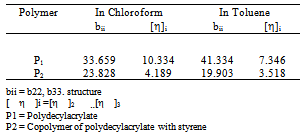
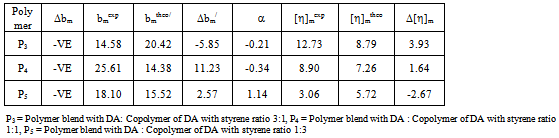
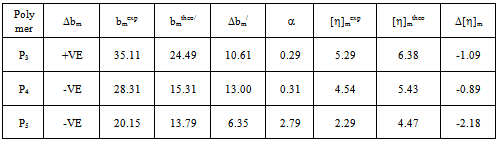
 values) except for P3 in toluene. According to this criterion there are repulsive intermolecular interaction existing between the polymers in ternary solutions. Here b23theo values were determined from b22 and b33 values for binary mixtures. But the hydrodynamic volume of polymers in ternary solution must be different from their binary solution because in ternary solution polymer-polymer interaction changes the effective hydrodynamic volume of the polymers. Again, in a ternary solution, the interactions involved are bothpolymer-polymer interaction and polymer-solvent interaction. Thus the solvents have an influence on bmexp values of the polymer blends.
values) except for P3 in toluene. According to this criterion there are repulsive intermolecular interaction existing between the polymers in ternary solutions. Here b23theo values were determined from b22 and b33 values for binary mixtures. But the hydrodynamic volume of polymers in ternary solution must be different from their binary solution because in ternary solution polymer-polymer interaction changes the effective hydrodynamic volume of the polymers. Again, in a ternary solution, the interactions involved are bothpolymer-polymer interaction and polymer-solvent interaction. Thus the solvents have an influence on bmexp values of the polymer blends.
|
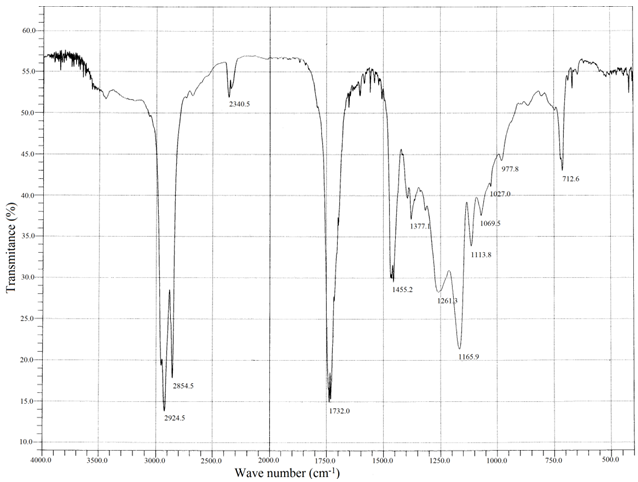 | Figure 1. FT-IR spectrum of poly decyl acrylate |
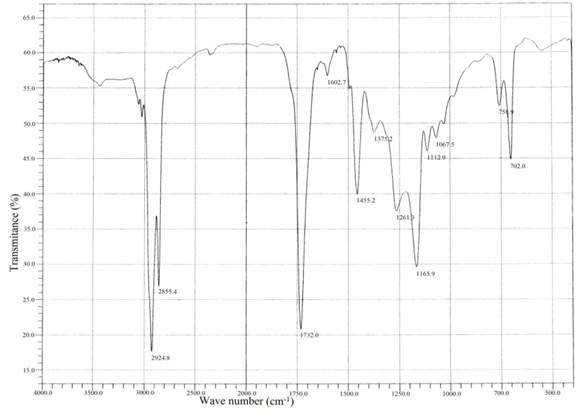 | Figure 2. FT-IR spectrum of poly decyl acrylate + Styrene |
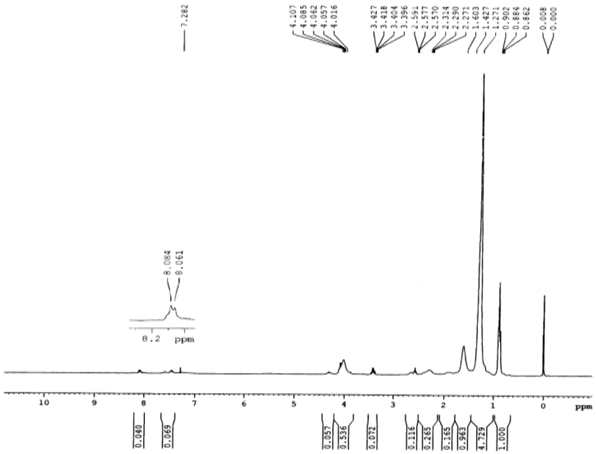 | Figure 3. 1H NMR spectrum of the copolymer of decyl acrylate with styrene |
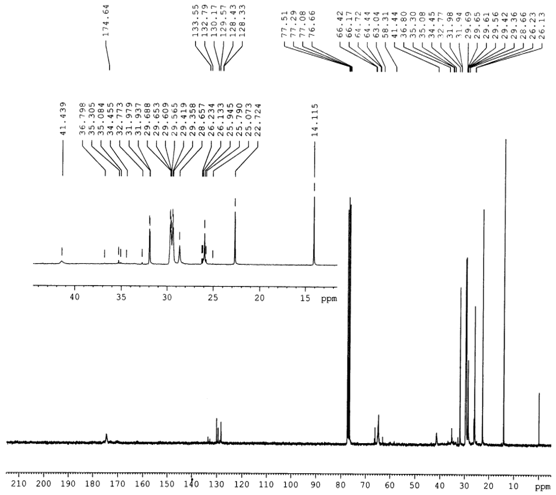 | Figure 4. 13C NMR spectrum of the copolymer of Decyl acrylate with styrene |
|
|
 values in both toluene and chloroform solutions except P3 in toluene. This is due to higher solubility of PDA in chloroform.In solutions of toluene, the theoretical
values in both toluene and chloroform solutions except P3 in toluene. This is due to higher solubility of PDA in chloroform.In solutions of toluene, the theoretical  values are lower than experimental values resulting negative
values are lower than experimental values resulting negative 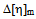 values for all blends. Again, in solutions of chloroform, at low (3:1) and equal (1:1) percentage of copolymer, theoretical
values for all blends. Again, in solutions of chloroform, at low (3:1) and equal (1:1) percentage of copolymer, theoretical 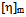 values are again lower than experimental values resulting negative
values are again lower than experimental values resulting negative 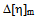 values indicating incompatibility of polymers in ternary solutions, but at higher (1:3) percentage of copolymer theoretical
values indicating incompatibility of polymers in ternary solutions, but at higher (1:3) percentage of copolymer theoretical 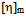 values are lower than experimental values resulting negative
values are lower than experimental values resulting negative 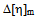 values which indicates compatibility of polymers in polymer blends. This is due to the fact that, here chloroform is act as better solvent than toluene and for this reason the polymer–solvent interaction is more effective than polymer-polymer interaction. In other words, due to prominent solvent effect (good solvation) the experimental
values which indicates compatibility of polymers in polymer blends. This is due to the fact that, here chloroform is act as better solvent than toluene and for this reason the polymer–solvent interaction is more effective than polymer-polymer interaction. In other words, due to prominent solvent effect (good solvation) the experimental 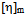 value increases with increasing hydrodynamic volume of polymer mixture and shows negative
value increases with increasing hydrodynamic volume of polymer mixture and shows negative 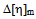 value. Again, literature indicate that solubility parameters of PDA and copolymer of DA with styrene are closer to chloroform than with toluene[24-28] but the polymers are in the solubility range of both solvents. Certain drop in
value. Again, literature indicate that solubility parameters of PDA and copolymer of DA with styrene are closer to chloroform than with toluene[24-28] but the polymers are in the solubility range of both solvents. Certain drop in 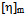 value for P5 in chloroform is due to dissimilar structure of chloroform and the copolymer with styrene.The forth compatibility parameter, α, gives, positive value for all three polymer blends in toluene. So polymer mixtures are compatible in toluene solutions. In chloroform, α>0 for P5 polymer blends indicating compatibility. But in case of polymer blends P3 and P4, α<0 which indicating incompatibility. This can also be shown in the graphical presentation (Fig. 5 and Fig. 6) that the fifth parameter, change of slope of the curve in the plot of reduced viscosity against concentration in ternary system gives linear graph for binary systems. But in cases of ternary systems certain positive deviation in slope of the curve has been observed. So according to the fifth parameter all polymer blends in both solvents are compatible.
value for P5 in chloroform is due to dissimilar structure of chloroform and the copolymer with styrene.The forth compatibility parameter, α, gives, positive value for all three polymer blends in toluene. So polymer mixtures are compatible in toluene solutions. In chloroform, α>0 for P5 polymer blends indicating compatibility. But in case of polymer blends P3 and P4, α<0 which indicating incompatibility. This can also be shown in the graphical presentation (Fig. 5 and Fig. 6) that the fifth parameter, change of slope of the curve in the plot of reduced viscosity against concentration in ternary system gives linear graph for binary systems. But in cases of ternary systems certain positive deviation in slope of the curve has been observed. So according to the fifth parameter all polymer blends in both solvents are compatible.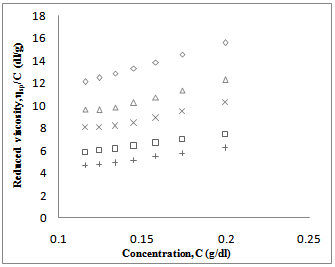 | Figure 5. Plot of reduced viscosity, vs. concentration C in chloroform; ◊, P1; □, P2; ∆, P3; ×, P4; +, P5 vs. concentration C in chloroform; ◊, P1; □, P2; ∆, P3; ×, P4; +, P5 |
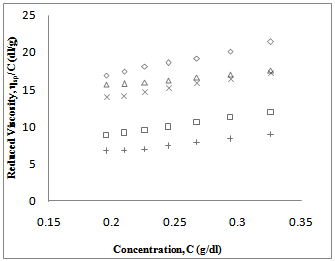 | Figure 6. Plot of reduced viscosity,  vs. concentration C in Toluene; ◊, P1; □, P2; ∆, P3; ×, P4; +, P5 vs. concentration C in Toluene; ◊, P1; □, P2; ∆, P3; ×, P4; +, P5 |
|
|
|
5. Conclutions
- Though both homo and copolymer of decylacrylate (DA) are efficient as viscosity index improver (VII) and pour point depressant (PPD), homopolymer of DA performed better as VII than copolymer. But copolymer of DA with styrene performed better as PPD than homopolymer.The polymer blends are compatible in chloroform and toluene and also in two different base oils. The compatibility of polymer mixtures was better in toluene than in chloroform. The compatibility of polymer mixtures also increases with increasing copolymer percentage.The efficiency of all polymer blends as PPD and VII were much better than the individual homo and copolymers due to compatibility. Polymer blends with higher copolymer percentage performed better as PPD and VII.With increasing concentration the efficiency of polymer blends as VII increases but their effectiveness as PPD decreases.
ACKNOWLEDGEMENTS
- Thanks are due to University Grants Commission, New Delhi for financial assistance and to IOCL for supplying the base oils used in the investigation.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML