-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Polymer Science
p-ISSN: 2163-1344 e-ISSN: 2163-1352
2012; 2(5): 129-134
doi: 10.5923/j.ajps.20120205.08
Modification of One-component Polyurethane by Organosilicone
V. Yu. Chukhlanov , M. Ionova
Department of Chemistry and Ecology, Vladimir State University, Vladimir, 600000, Russia
Correspondence to: V. Yu. Chukhlanov , Department of Chemistry and Ecology, Vladimir State University, Vladimir, 600000, Russia.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
In the article it’s considered an interreacting of polyurethane prepolymer and organosilicone – polymethylphenylsiloxane – to get a protecting paint coating with improved operating properties. It’s studied the influence of modifier on surface structure of cured coating.
Keywords: Polyurethane, Organosilicone, Polymethylphenylsiloxane, Protecting Coating
Article Outline
1. Introduction
- The using of polyurethane polymer coatings as protecting coatings is of great interest now for the most of science and technical fields. This polymer materials have high processing and operating properties.However, in spite of good properties these coatings are unstable to influence of many atmosphere and anthropogenic factors. The UV-light’s exposure, atmosphere ozone, regular temperature drop, temperature expansion, a freezing of moisture inside of material and etc. cause to destruction.Besides of them, polyurethane coatings have some operating negatives, such as high moisture absorption, low high-temperature resistance. In this regard, the interest is to use modifiers, such as organic silicone, for polymers to get block-copolymers or interpenetrating nets[1, 2, 3]. Organic silicone compounds and modified organic polymers by silicones have high resistance not only to high temperatures and UV-light but to atmosphere moisture also due to the strongest hydrophobic effect appeared at such modification [4, 5].The goal of present work is to research physical, mechanical and operating properties of polymer coating based on one-component polyurethane compound modified polyorganosiloxane.
2. Objects and Methods of Researching
- In earlier published works[2, 4] unfilled protectingcompositions with hydrophobic effect based on one-component polyurethane lacquer modified by alkoxysilane are considered. Since 2007 a pilot operation of such compounds has shown a high effectiveness of the material. As a polyurethane component, it was selected a polyurethane prepolymer based on polyether synthesized from glycerin, ethylene oxide and propylene oxide, and polyisocyanate. The content of free NCO – groups in synthesized prepolymer is 19%, the dynamic viscosity is 4600mPa*s. This prepolymer was selected to get the best properties of cured coating. The big amount of hard segments in the structure of polyisocyanate and its ability to crystallize give additional hardness to cured coating and improve its resistance to abrasive wear. The polyol consists of short segments of chain and has soft-segment structure. The polyol is in noncrystalline state and able to long relaxation strains due to big amount of intramolecular hydrogen bonds[6].
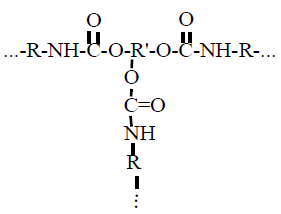 As a modifier, it was selected a silicon polymer – a polyorganosilicone, with reactive hydroxyl groups in a side chain and which has high heat resistance (up to 300℃) and low water absorption. The ionic character of siloxane bond, which requires high activation energy, gives the molecule a special resistance to high temperature:polymethylphenylsiloxane keeps the operating data during long time at 300℃. A small rotation limit and long chemical bonds allow siloxane to be in different conformations. It causes to increasing of surface tension of whole film because methyl and phenyl radicals are found on the macromolecule surface.
As a modifier, it was selected a silicon polymer – a polyorganosilicone, with reactive hydroxyl groups in a side chain and which has high heat resistance (up to 300℃) and low water absorption. The ionic character of siloxane bond, which requires high activation energy, gives the molecule a special resistance to high temperature:polymethylphenylsiloxane keeps the operating data during long time at 300℃. A small rotation limit and long chemical bonds allow siloxane to be in different conformations. It causes to increasing of surface tension of whole film because methyl and phenyl radicals are found on the macromolecule surface. 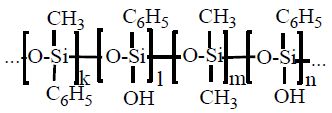 To define the polyurethane prepolymer modification effect by polymethylphenylsiloxane it’s necessary to research the structure of polymer film surface before and after modification. To research nanostructure of films it was applied the atomic force probe microscopy. In order to research a structure of chemical bonds it was used the Fourier infrared spectrophotometer Avatar 360 FT-IR ESP. The water absorption of modified and non-modified films were defined according to GOST 2678-94, the defining of wetting angle according to GOST 7934.2-74, an adhesion on the glass – by using of adhesiometer PSO – MG4 according to GOST 28574-90, relative hardness by using of rocker M-3 according to GOST 5233-89. The structure of surface was researched by scanning probe device Integra Aura, made by NT-MDT, Zelenograd, Russia. The scanning was made by polysilicon probes HA_NC at intermittent contact method. At using of this method the pressure of cantilever on prototype surface is less and it allows to work with softer materials such as polymer films and biomaterials. cantilever cantilever cantilever cantileverThe coverings of surface by compositions for experiments were made at standard environment: at 20℃, atmosphere pressure 0.1MPa, relative air humidity 75%. To cover it was used a special applicator with adjustable slit. The compound was covered the mirror glass by such applicator. The thickness if cured coating is 50 mkm.
To define the polyurethane prepolymer modification effect by polymethylphenylsiloxane it’s necessary to research the structure of polymer film surface before and after modification. To research nanostructure of films it was applied the atomic force probe microscopy. In order to research a structure of chemical bonds it was used the Fourier infrared spectrophotometer Avatar 360 FT-IR ESP. The water absorption of modified and non-modified films were defined according to GOST 2678-94, the defining of wetting angle according to GOST 7934.2-74, an adhesion on the glass – by using of adhesiometer PSO – MG4 according to GOST 28574-90, relative hardness by using of rocker M-3 according to GOST 5233-89. The structure of surface was researched by scanning probe device Integra Aura, made by NT-MDT, Zelenograd, Russia. The scanning was made by polysilicon probes HA_NC at intermittent contact method. At using of this method the pressure of cantilever on prototype surface is less and it allows to work with softer materials such as polymer films and biomaterials. cantilever cantilever cantilever cantileverThe coverings of surface by compositions for experiments were made at standard environment: at 20℃, atmosphere pressure 0.1MPa, relative air humidity 75%. To cover it was used a special applicator with adjustable slit. The compound was covered the mirror glass by such applicator. The thickness if cured coating is 50 mkm.3. Results of Experiments
- At first step of experiments it were supposed a possible way of one-component polyurethane andpolymethylphenylsiloxane interaction and a possibility of chemical bonds between these materials forming due to existing of reactive NCO-groups in polyurethane and hydroxyl groups of polymethylphenylsiloxane. Most likely the product of prepolymer and polymethylphenylsiloxane interaction is a third-dimensional cross-linked polymer with cross-linked polyurethane macromolecules by organosilicone. The isocyanate groups and hydroxyl siloxane groups interaction are in the basis of this chemical reaction[5, 6, 7]. The reaction of forming third-dimensional cross-linked structures between polyurethane and polymethylphenylsiloxane, probably, goes according to following way:Due to high reactivity in parallel to main above mentioned reaction it goes other co-reactions: polyisocyanate interaction with water, carbamide and urethane group contained combinations[8]. The nature and the structure of synthesized chemical bonds are determinated by the speed of reaction[9]. Among the co-reactions, the curing reaction of NCO-groups of polyurethane prepolymer by air moisture due to simple structure of water molecule has the highest speed[10].According to made calculations with MathCAD the effective constant of reaction polyurethane prepolymer and polymethylphenylsiloxane is 0.399 l/mole-rat*sec, the constant of reaction polyurethane prepolymer and air moisture is 0.378 l/mole-rat*sec. Consequently, reactions of isocyanate group with polymethylphenylsiloxane hydroxyl groups and water go with almost the same speed[11].To confirm our supposition about Si – О – С chemical bonding in the macromolecule it were done researches of the polymer structure by Fourier infrared spectrophotometer. It was found peaks specified for existing of this type of bonds.
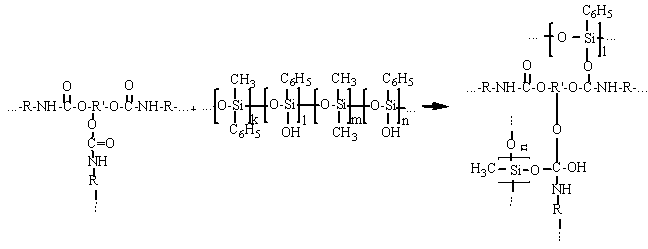 (1)
(1)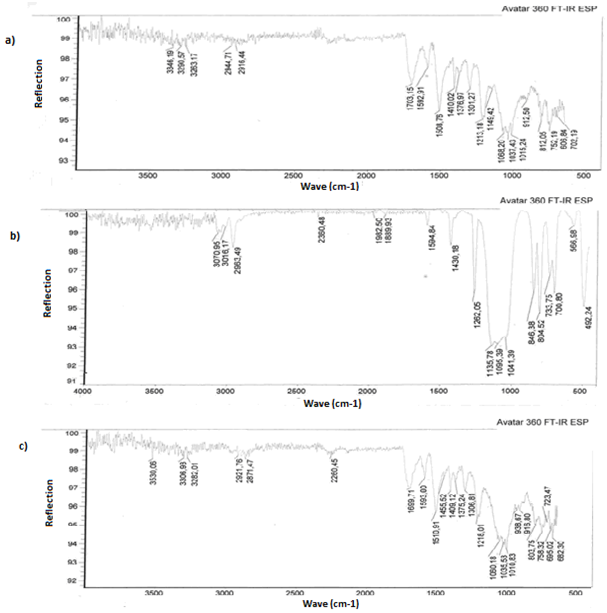 | Figure 1. Comparative infrared spectra of non-modified and modified polyurethane |
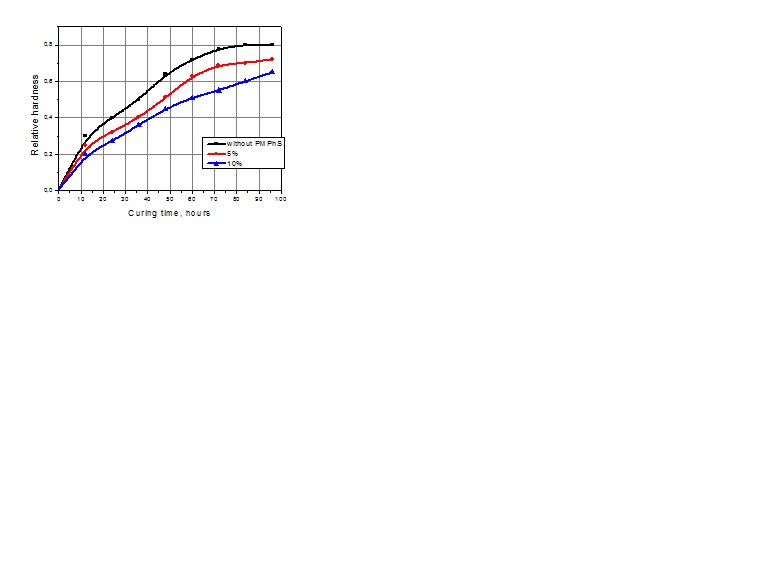 | Figure 2. Influence of hardening time content of modifier on relative hardness of coating |
 | Figure 3. Microphotography of compound’s hydrophobic properties defining а) without modifier; b) with modifier (20%) |
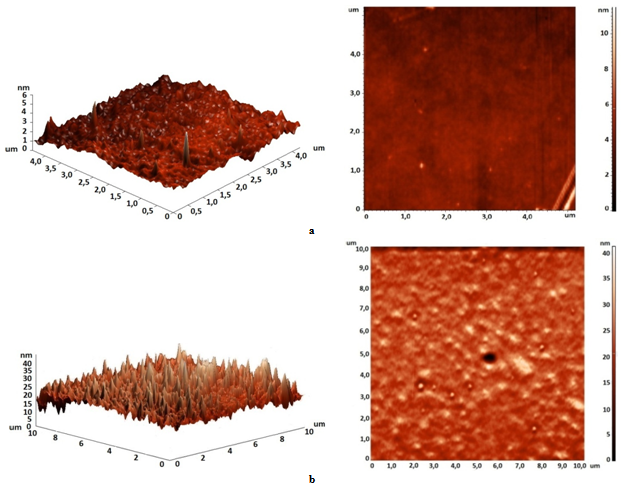 | Figure 4. Surface structure of non-modified polyurethane film (a) and polyurethane modified by ten percents polymethylphenylsiloxane (b) |
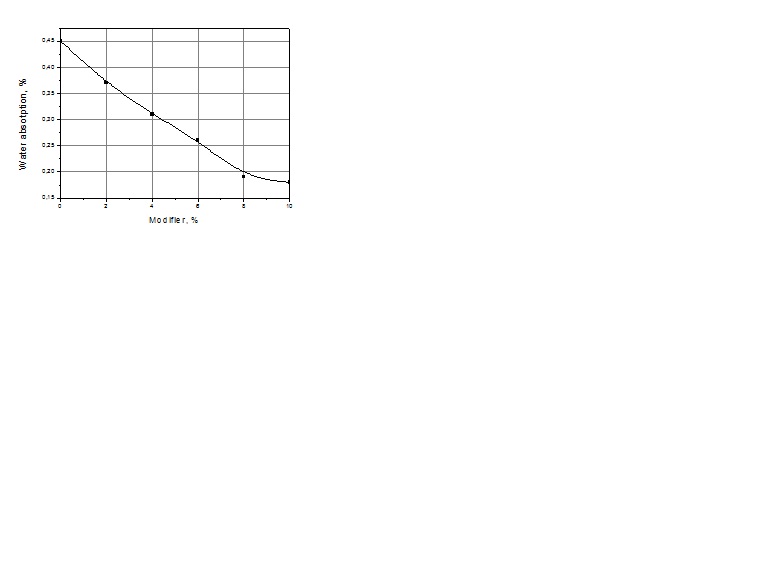 | Figure 5. Water absorption of modified polyurethane coating |
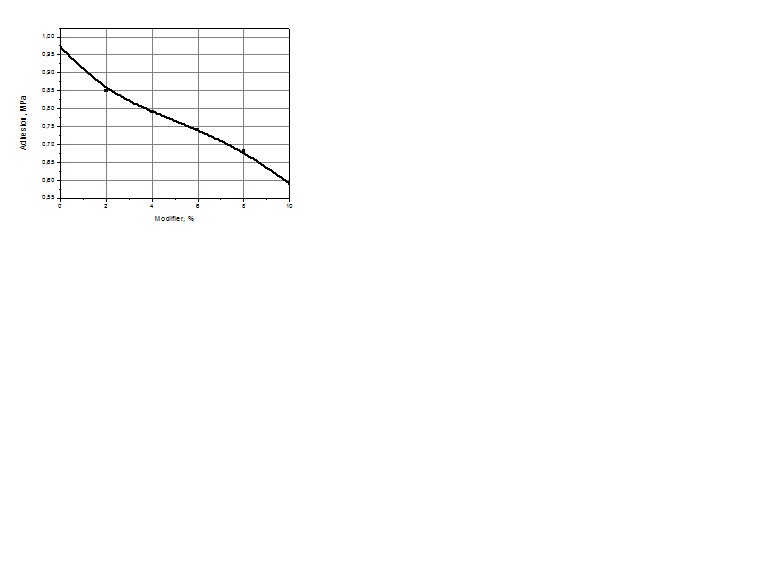 | Figure 6. Dependence of tear strength on modifier |
4. Conclusions
- The made researches have shown that modified polyurethane one-component lacquer successfully can be applied to protect constructions and buildings against negative natural and anthropogenic factors. The chemical modification of polyurethane allows to change its properties widely in necessary direction without decline other its data.
References
| [1] | V. Yu. Chukhlanov, N. A. Kolysheva, “New polymer binders based on oligopiperylene styrene and alkoxysilanes”, International Polymer Science and Technology, 35, №1, 2008. |
| [2] | V. Yu. Chukhlanov, M. Ionova, “Polyurethane coating modified by alkoxisilan with improved working properties”, Stroitelnie materiali, №4, pp. 60-61, 2012. |
| [3] | E. R. Volkova, V. V. Tereshatov, “Researching of opportunity to apply filled polyurethane materials as protective coatings”, Plasticheskie massi, №5, pp. 39-41, 2009. |
| [4] | V. Yu. Chukhlanov, M. Ionova, “Modification of protective polyurethane coating by tetraethoxysilane”, V International Conference on students and post-graduated students “Chemistry in the modern world”, Sankt-Peterburg, pp. 325-326, 2010. |
| [5] | Aziz M. Muzafarov, “Silicon Polymers”, Springer-Verlag Berlin Heidelberg, p. 100, 2011. |
| [6] | P. Sykes, “A guidebook to mechanism in organic chemistry”, New York: Longman Scientific & Technical, p. 247, 1991. |
| [7] | Arun ANAND PRABU and Muthukaruppan ALAGAR, “Mechanical and electrical studies of silicone modified polyurethane-epoxy intercrosslinked networks”, Polymer Journal, Vol. 36, №10, pp. 848-855, 2004. |
| [8] | M. Bock, “Polyurethane for coating”, Vincentz Vertrag, pp. 1-215, 2001. |
| [9] | A. I. Kurkin, A. B. Kulikov, “Influence of nature of polyol on general properties of polyetherurethanediols”, Stroitelnie materiali, №6, pp. 20-21, 2008. |
| [10] | V. A. Yamskiy, V. A. Koftyuk, “Influence of hydroxyl containing oligomers on the properties of two-components polyurethane paints”, Lakokrasochnie materiali I ih primenenie, p.14, №6, 2008. |
| [11] | B. A. Izmaylov, V. A. Vasnev, “Modification of material surface by functional organosiloxane”, Plasticheskie massi, pp. 58-61, №6, 2011. |
| [12] | M. A. Yarmolenko, A. A. Rogachev, A. V. Rogachou, D. L. Gorbochev, “Kinetic characteristics of dispersion of organosilicon compounds in vacuum and molecular structure of the coatings, deposited from volatile products of dispersion”, Problemi physiki, matematiki i techniki,pp. 32-38, №3(8), 2011. |
| [13] | V. Yu. Chukhlanov, M. Ionova, “Reducing water absorbtion of polyurethane coating”, International scientific and practical Conference “Modern directions of theoretical and practical researchings 2012”, Odessa, pp.85-87, 2012. |
| [14] | M. L. Kerber, V. M. Vinogradov, “Polymer compounds: structure, properties, technology”, Professiya, pp. 37-39, 2008. |
| [15] | M. Yu. Dolomatov, M. Yu. Timofeeva, “Theory of macromolecular compounds’s adhesion and its practical using”, Plasticheskie massi, pp. 45-47, №3, 2009. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML