-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Polymer Science
p-ISSN: 2163-1344 e-ISSN: 2163-1352
2012; 2(5): 79-84
doi:10.5923/j.ajps.20120205.01
Poly(vinyl alcohol)-Salicylic Acid Conjugate: Synthesis and Characterization
Roman Jantas, Zbigniew Draczyński, Lucyna Herczyńska, Dawid Stawski
Department of Physical Chemistry of Polymers, Technical University of Łódź, 90-924, Łódź, Poland
Correspondence to: Roman Jantas, Department of Physical Chemistry of Polymers, Technical University of Łódź, 90-924, Łódź, Poland.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The polymeric system containing hydrolysable ester bonds linked to salicylic acid was synthesized for controlled drug release. Poly(vinyl alcohol) (PVA) functionalized with chloroacetate groups was obtained by reaction of PVA with chloroacetyl chloride using the N,N-dimethylacetamide/5% lithium chloride system as solvent and pyridine as catalyst. The degree of substitution was calculated from the chloride content and ranged from 37.8 to 98.9 mol%. The coupling of salicylic acid to PVA functionalized with chloroacetate groups was carried out by the reaction with between PVA and the sodium salt of salicylic acid. The structures of chloroacetylated PVA and PVA-salicylic acid conjugates were determined by means FTIR, 1H-NMR and 13C-NMR spectra. The hydrolysis in the heterogeneous system of PVA-salicylic acid conjugates were performed in buffer solutions (pH 7.6 and 8.5) at 37℃. Detection of hydrolysis by UV spectroscopy showed that released the drug, can by achieved by the hydrolysis of the ester bond between the drug and the polymeric carrier. The release profiles indicated that the release of the drug (sodium salicylate) from tablets was dependent on hydrophilic character of conjugate and the pH of the buffer solution.
Keywords: Poly(Vinyl Alcohol), Chloroacetylation, Sodium Salicylate, Polymer-Drug Conjugate, Controlled Release
Cite this paper: Roman Jantas, Zbigniew Draczyński, Lucyna Herczyńska, Dawid Stawski, Poly(vinyl alcohol)-Salicylic Acid Conjugate: Synthesis and Characterization, American Journal of Polymer Science, Vol. 2 No. 5, 2012, pp. 79-84. doi: 10.5923/j.ajps.20120205.01.
Article Outline
1. Introduction
- In the last decades much attention has been directed to speciality polymers for biomedical application. One particular approach towards the improved use of drugs is the design of polymer-drug conjugates or polymeric prodrugs[1-9]. The chemical attachment of low molecular weight drugs to synthetic or natural polymers has been frequently investigated as a means of improving the efficacy of drug control release devices through a constant but prolonged release of drugs with minimum side effects. The drugs may be linked to the polymeric carriers using a number of chemical reactions with participation of functional groups such as hydroxyl or carboxyl which are either originally present in the polymer chain or alternatively formed by functionalization. Another possibility is the use of functionalized monomers in synthesis of a reactive polymeric precursor. In most cases, drugs bound directly to the polymer chain exhibit either a reduced or zero biological activity. For this reason, drugs should be separated from the polymeric backbone by means of a spacer. Once the drug conjugate reaches the target compartment, the drug can then be split off more readily in its active form. To facilitate the release of the drug it must be attached to the macromolecu lar chain by covalent bonds of limited stability in a biological environment.The advantages of poly(ethylene oxide), poly(vinyl pyrrolidone), poly(2-hydroxypropyl methacrylamide), copolymers of 2-hydroxyethyl methacrylate or polysaccharides as a macromolecular carrier for drug immobilization are well acknowledged, as is apparent from the literature data[10-19]. In most cases the polymers has been previously transformed into a suitable reactive derivative, in order to achieve the attachment of drug molecules and to introduce a spacer between the carrier and the bioactive compounds. Selection of the spacer arm is critical as it opens the possibility of controlling the site and the rate of release of the drug from the conjugates either by hydrolytic or enzymatic cleavage of the linking bond.Some natural or synthetic polymers possess multiple primary and secondary hydroxyl groups and there can be easily conjugated with drug molecules with reactive groups either by direct conjugation or by incorporation of a spacer arm. In this paper, PVA with reactive primary hydroxyl groups may be used as a polymeric carrier for coupling pharmaceutical compounds.Salicylates are used in medicine as analgesic and antipyretic agents. They also act as non-steroidalanti-inflammantory drugs (NSAIDs) and induces apoptosis in cancer cells [20-23]. Recent years have witnessed many studies on the synthesis and hydrolysis of polymer-drug conjugates of NSAIDs such as: naproxen[12,24] indomethacin[10,25,26] ibuprofen[27,28] ketoprofen[12,29] and fenoprofen[30-32].The aim of the present paper was to synthesize and characterize PVA-salicylic acid conjugates in a two-stage procedure. During the first stage, PVA was chloroacetylated with chloroacetyl chloride, while in the second stage, chloroacetate groups were reacted with sodium salicylate. A study of the hydrolysis of the resulting conjugates in the heterogeneous system was also conducted in aqueous buffer solutions (pH 7.6 and 8.5) and the quantity of the released drug was detected by UV spectroscopy. The influence of neighbouring groups on the release of the drug from the conjugates was also studied.
2. Experimental
2.1. Materials
- Poly(vinyl alcohol) (PVA) was commercial product (Aldrich). The average molecular weight of PVA was MW=31.600-50.000 g/mol, 98-99% hydrolyzed. N,N- dimethylacetamide (DMAc) (Aldrich), dimethylsulfoxide (DMSO) (Aldrich) was purified by distillation and then stored over 4 Å moleculare sieves. Lithium chloride (LiCl) (Aldrich) was dried under reduced pressure in the presence of phosphorus pentoxide. Chloroacetyl chloride (Aldrich) was purified before use by distillation under reduced pressure. Pyridine (POCh) was refluxed over CaH2 under a nitrogen atmosphere and then distilled. Salicylic acid (SA) (Fluka, Buchs, Switzerland) was used without further purification. Sodium salicylate (SSA) was obtained by dissolving 8.05 g (0.05 mol) of the acid in 50 ml of chloroform, then neutralized with 2.0 g (0.05 mol) of NaOH dissolved in 50 ml of ethyl alcohol. The product precipitated by pouring reaction mixture into 600 ml of dry acetone. After filtration, the salt was dried under reduced pressure at 50℃ to constant weight.
2.2. Procedures
2.2.1. Esterification of PVA with Chloroacetyl Chloride
- The typical procedure, of the esterification was as follow: 2.2 g (50.0 mmol, OH groups) of PVA was dissolved in 30 ml DMAc/ 5%LiCl solvent system. The solution was then charged into a three-necked flask equipped with a nitrogen inlet and outlet, dropping funnel, magnetic stirrer and thermometer. Pyridine 4.68 ml (60.0 mmol) was added to the flask as an acid acceptor. DMAc solution (10 ml) containing chloroacetyl chloride 5.76 ml (60.0 mmol) was then added dropwise at about 0℃ with stirring. The reaction mixture was heated at 25℃ for 8 h and after the solution was poured into a large amount of cold 2M HCl to precipitate the product. The precipitated product was filtered and washed several times with cold distilled water. It was purified by reprecipitation using THF as solvent and cold distilled water as precipitant, then dried under reduced pressure at 50℃ to constant weight. The yield was 79%.
2.2.2. Reaction of Chloroacetylated PVA with Sodium Salicylate
- The typical procedure, of the reaction was as follow: The chloroacetylated PVA 2.1 g (19.5 mmol ClCH2CO- groups) was dissolved in 40 ml DMSO at room temperature and then 3.68 g (23 mmol) sodium salicylate was added while stirring. The reaction was performed at 30℃ and under stirring for about 5 h. The obtained product was isolated by precipitation using distilled water as precipitant and then ethanol washed to remove unreacted sodium salicylate. All samples were purified by reprecipitation, using DMSO as solvent and ethanol as precipitant and then dried under reduce pressure at 60℃ to constant weight. The yield was 69%.
2.2.3. Study of The Heterogeneous Hydrolysis of PVA-Salicylic Acid Conjugate
- Samples of the PVA-salicylic acid conjugate, about 0.1 g (containing from 37.8 to 98.9 mol-% salicylate groups) in the form of powder were pressed in steel cylindrical cell with a diameter of 12 mm in a hydraulic press under a pressure of about 12 MPa to make disks. The resulting tablet was placed in conical flasks with 100 ml of an aqueous buffer solution (pH 7.6 and 8.5). The flaskes were put into water bath heated to 37℃. 2 ml samples were taken at appropriate intervals from the liquid above of the tablets and equal volume of same dissolution medium was added to maintain a constant volume. The solution contained the released drug, which was quantitatively determined by UV spectroscopy at the absorption wavelength of sodium salicylate (
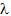 295 nm) using calibration curves obtained previously under the same conditions. Tests were performed for different degrees of substitution of the conjugates and various pH values of the reaction medium. We noticed that of PVA-salicylic acid conjugates remained insoluble in the reaction environment along the whole hydrolysis process investigated.
295 nm) using calibration curves obtained previously under the same conditions. Tests were performed for different degrees of substitution of the conjugates and various pH values of the reaction medium. We noticed that of PVA-salicylic acid conjugates remained insoluble in the reaction environment along the whole hydrolysis process investigated. 3. Measurements
3.1. Spectroscopic Measurements
- Infrared spectra were recorded using Perkin-Elmer 2000 (FTIR) instrument. 1H-NMR and 13C-NMR spectra were obtained using Bruker DPX 250 MHz spectrometer with DMSO-d6 as solvents and TMS as an internal reference. The UV-VIS spectra were obtained using Perkin Elmer UV/VIS Lambda 2 spectrometer.
3.2. Evaluation the Degree of Esterification
- The degree of the esterification of the PVA was determined from the elemental analysis of chloride. Elemental analysis (Cl) was carried out on a Carlo Erba 1106 EA-instrument.
4. Results and Discussion
- In order to provide a uniform distribution of chloromethyl groups along the polymer chain, the esterification was carried out in homogeneous system, previously dissolving PVA in DMAc/5% LiCl system. PVA modified with chloroacetate group with different degress of substitution was synthesized using the method described for chloroacetylation of starch [19] according to the reaction presented by the Scheme 1.
 | Scheme 1. Reaction between PVA and chloroacetyl chloride |
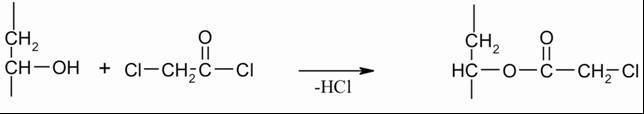
 | Scheme 2. Synthesis of PVA-salicylic acid conjugate |
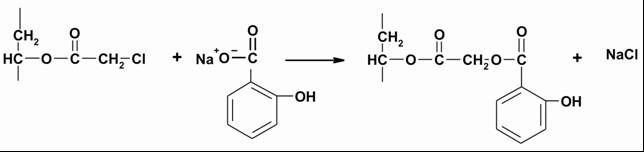
 | Figure 1. FTIR spectra of: a - PVA, b - chloroacetylated PVA (98.9 mol% of chloroacetate groups), c - conjugate of PVA-salicylic acid (98.9 mol% of salicylate groups) |
 | Figure 2. 1H-NMR spectra of: a - chloroacetylated PVA (98.9 mol% of chloroacetate groups), b - conjugate of PVA-salicylic acid (98.9 mol% of salicylate groups) |
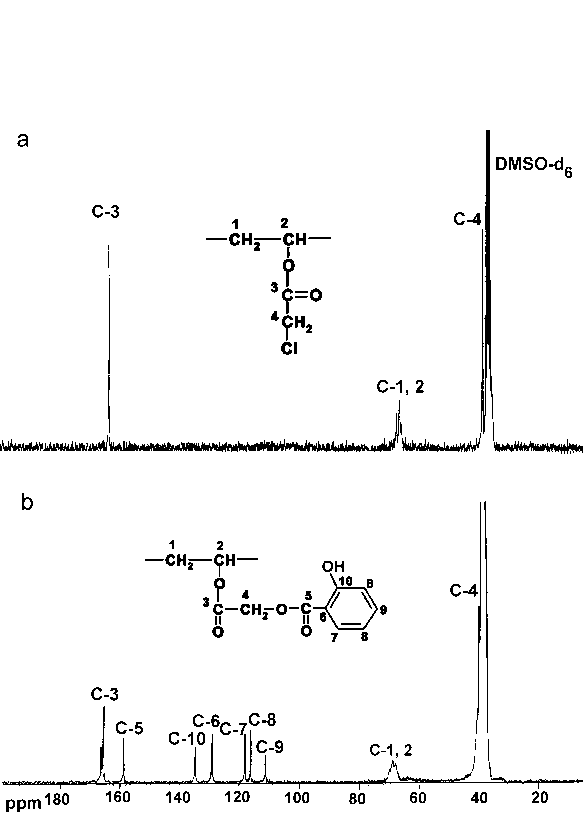 | Figure 3. Spectra 13C-NMR of: a - chloroacetylated PVA (98.9 mol% of chloroacetate groups), b - conjugate of PVA-salicylic acid (98.9 mol% of salicylate groups) |
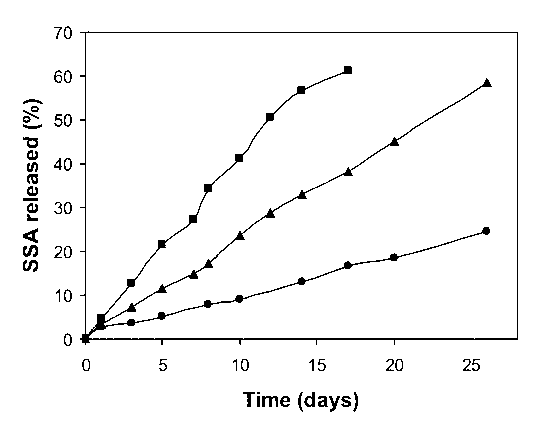 | Figure 4. The release of the sodium salicylate with conjugate of PVA-salicylic acid depending on conjugate compositions: (■) 37.8 mol% salicylate groups; (▲) 73.6 mol% salicylate groups; (•) 98.9 mol% of salicylate groups (pH 8.5 at 37℃) |
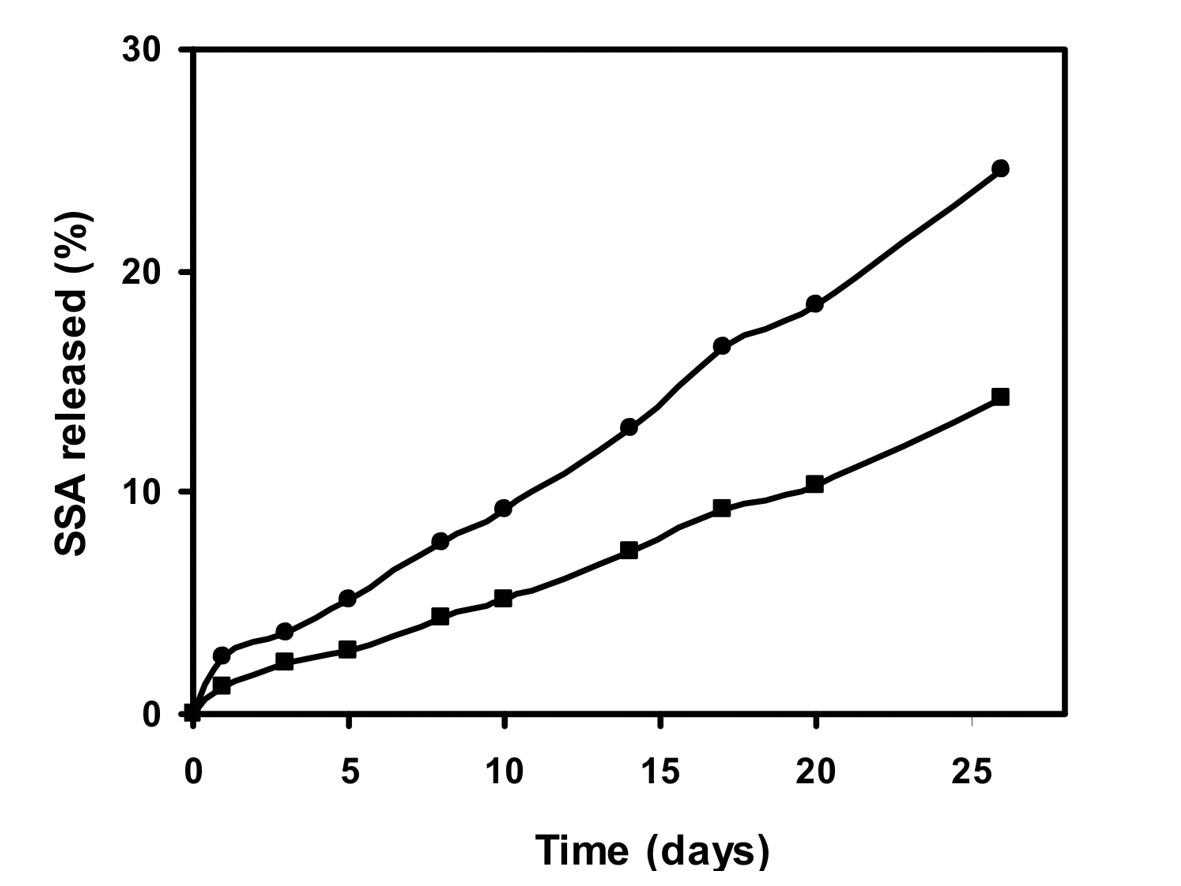 | Figure 5. The release of the sodium salicylate with conjugate of PVA-salicylic acid depending on pH value of reaction environment: (■) pH 7.6, (•) pH 8.5 (98.9 mol% salicylate groups, at 37℃) |
5. Conclusions
- The studied performed have shown that as a result of the reaction between PVA functionalized with chloroacetate groups and sodium salts of salicylic acid a new conjugate PVA-salicylic acid is obtained. The chemical structures of all the modification products of PVA were assessed by means of FT-IR , 1H-NMR and 13C-NMR spectroscopy. The hydrolysis of the conjugates was examined under physiological conditions. The results obtained have shown that the procentage of the drug resleased increases with increasing conjugate hydrophilicity and pH of buffer solutions. The results suggest that the PVA-salicylic acid conjugate can be a useful carrier for controlled release of the drug.
References
| [1] | Khandare, J.; Minko, T.; Polymer-drug conjugates: Progress in polymeric prodrugs. Prog. Polym. Sci. 2006, 31, 359-397 |
| [2] | Hoste, K.; De Winne, K.; Schacht, E. Polymeric prodrugs. Int. J. Pharm. 2004, 277, 119-131 |
| [3] | Kim, I.S.; Kim, S.H. Development polymeric nanoparticulate drug delivery system: In vitro characterization of nanoparticles based on super-containing conjugates. Int. J. Pharm. 2002, 245, 67-73 |
| [4] | Chang, J.; Du, J.; Zheng, Y. Synthesis and characterization of novel PGA and PLA prodrugs with sulfadiazine and 5-fluorouracil terminal groups. J. Macromol. Sci. 2007, 44, 887-892 |
| [5] | D′Souza, A.J.M.; Schowen, R.L.; Topp, F.M. Poly(vinyl pyrrolidone)-drug conjugate: synthesis and release mechanism. J. Control. Release 2004, 94, 91-100 |
| [6] | Hang, R.; Kratz, F. Polymer terapeutics: Concepts and applications. Angew. Chem. Int. Ed. 2006, 45, 1198-1215. |
| [7] | Babazadeh, M.; Synthesis and study of controlled release of ibuprofen from the new acrylic type polymers. Int. J. Pharm. 2006, 316, 68-73 |
| [8] | Liu, Z.; Rimmer, S. Synthesis and release of 5-fluorouracil from poly(N-vinylpyrrolidone) bearing 5-fluorouracil derivatives. J Control. Release 2002, 81, 91-99 |
| [9] | Dvorak, M.; Kopečkova, P.; Kopeček, J. High-molecular weight HPMA copolymer-adriamycin conjugates. J. Control. Release 1999, 60, 321-322 |
| [10] | Bonina, F.P.; Motenegro, L.; Capraiis, P.D.; Palagiano, F.; Trapani, G.; Liso, G. In vitro and in vivo evaluation of polyoxyethylene indomethacin ester as dermal prodrugs. J. Control. Release 1995, 34, 223-232 |
| [11] | San Roman, J.; Levenfeld, B. Polymers with pharmacological activity. Macromolecules 1990, 23, 423-427 |
| [12] | Bonina, F.P.; Puglia, C.; Barbuzzi, T.; Capraiis, P.D.; Palagiano, F.; Rimoli, M.G.; Saija, A. In vitro and in vivo evaluation of polyoxyethylene ester as dermal prodrugs of ketoprofen, naproxen and diclofenac. Eur. J. Pharm. Sci. 2001, 14, 123-134 |
| [13] | Babazadeh, M. Synthesis, characterization and in vitro drug-release properties of 2-hydroxyethyl methacrylate copolymers, J. Appl. Polym. Sci. 2007, 104, 2403-2409 |
| [14] | Sanchez-Chaves, M.; Arranz, F.; Diaz, C. Controlled release behaviour of 2-acetoxybenoic acid-dextran conjugates. Macromol. Chem. Rapid. Commun. 1989, 10, 431-433 |
| [15] | Duarte, A.R.; Costa, M.S.; Simplicio, A.L.; Cardoso, M.; Duarte, C.M.M. Preparation of controlled release microspheres using supercritical fluid technology for delivery of anti-inflammatory drugs. Int J. Pharm. 2006, 308, 168-174 |
| [16] | Won, Ch.Y.; ChCh, Chu.; T, Yu. Synthesis of starch-based drug carrier for the control/release of estrone hormone. J. Carbohydrate Research 1997, 32, 239-244 |
| [17] | Sanchez-Chaves, M.; Arranz, F.; Diaz, C. Synthesis and characterization of 2-acetoxybenzoic acid-dextran ester conjugates. Macromol. Chem. 1989, 190, 2391-2396 |
| [18] | Davaran, S.; Hanaee, J.; Khosravi, A.; Release of 5-amino salicylic acid from acrylic type polymeric prodrugs designed for colon-specific drug delivery. J. Control. Release 1999, 58, 279-287 |
| [19] | Jantas; R.; Draczyński, Z.; Stawski, D. Starch functionanalized by chloroacetate groups: coupling of bioactive salicylic acid. Starch, 2007, 59, 366-370 |
| [20] | Clarc W.G.; Brater D.C.; Johnson A.R. Goth's Medical Pharmacology, 12 edn. C.V. Mosby Company. St. Louis Washington D.C. 1988 |
| [21] | San Roman, J.; Madruga E.L.; Pargoda L. Synthesis and microstructure of polymers from o-methacryloyloxybenzoic acid. J. Polym. Sci. Polym. Chem. Edn. 1987, 25, 203-214 |
| [22] | Cai, Q.; Zhu, K.J.; Zhang, J. Salicylic acid and PEG-contained polyanhydrides: synthesis, characterization and in vitro salicylic acid . J. Drug Delivery. 2005, 12, 97-102 |
| [23] | El-Refaie Kenawy, Salem S. Al-Deyab, Mohamed H. El-Newehy, Controlled release of 5-aminosalicylic acid (5-ASA) from new biodegradable polyurethanes. |
| [24] | Molecules 2010, 15, 2257-2268 |
| [25] | Davaran, S.; Entezami, A.; Acrylic type polymers containing ibuprofen and indomethacine with difunctional spacer group: synthesis and hydrolysis. J. Control. Release 1997, 47, 41-49 |
| [26] | Kim, S.Y.; Shin, I.G.; Lee, Y.M.; Cho, C.S.; Sung, Y.K. Methoxy poly(ethylene glycol) and ε-caprolactone amphiphilic block copolymeric micelle containing indomethacen: II Micelle formation and drug release behaviours. J. Control. Release 1998, 51, 13-22 |
| [27] | Davaran, S.; Entezami, A. Hydrophilic copolymers prepared from acrylic type derivatives of ibuprofen containing hydrolyzable thioester bond. Eur. Polym. J. 1998, 34, 187- 192 |
| [28] | Chang, C.H.; Sheu, Y.M.; Hu, W.P.; Wang, L.F.; Chen, J.S. Synthesis and properties of copolymers 2-hydroxyethyl methacrylate-linked nonsteroidal ant-inflammatory agents with methacrylic acid. J. Polym. Sci. Polym. Chem. 1998, 36, 1481-1490 |
| [29] | Zovko, M.; Zorc, B.; Lovrek, M.; Boneschenans, B. Macromolecular prodrugs IX Synthesis of polymer- fenoprofen conjugates. Int. J. Pharm. 2001, 228, 129-138 |
| [30] | Kim, S.Y.; Chung, C.W.; Hwang, S.J.; Rhee, Y.H. Drug release from and hydrolytic degradation of a poly(ethylene glycol) grafted poly(3-hydroxyoctanoate). Int. J. Biol. Macromol. 2005, 36, 84-89 |
| [31] | Merwe, T.V.D.; Boneschenans, B.; Zorc, B.; Breytenbach, J.; Zovko, M. Macromolecular prodrugs. X Kinetics of fenoprofen release from PHEA-fenoprofen conjugate. Int. J. Pharm. 2002, 241, 223-239 |
| [32] | Namazi, B.; Babazadeh, M. ; Sarabi, A.; Entezami, A.Synthesis and hydrolysis of acrylic type polymers containing nonstroidal antyinflammantory drugs. J. Polym. Mater. 2001, 18, 301-312 |
| [33] | Omata, K.; Nozawa, Y.; Higashide, F. Drug released from cellulose acetic phthalate gel, J. Appl. Polym. Sci. 1977, 21, 2009-2012 |
| [34] | Harris, F.W. In Medical Application of Controlled Release. Langer, RS.; Wise, D.L.; Eds.; CRC Boca Raton FL. 1984, p 103 |
| [35] | Levenfeld, B.; San Roman, J.; Madrug, E. Polymers with farmacological activity: Synthesis and free radical polymerization of an acrylic derivative of phenacetin. Polymer 31(1990) 160-164 |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML