-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Polymer Science
p-ISSN: 2163-1344 e-ISSN: 2163-1352
2012; 2(4): 73-78
doi: 10.5923/j.ajps.20120204.05
Modification and Characterization of Polyacrylic Acid for Metal Ion Recovery
Magdy Y. Abdelaal 1, 2, Mohammad S.I. Makki 1, Tariq R.A. Sobahi 1
1Chemistry Department, Faculty of Science, King Abdulaziz University, PO Box 80203, Jeddah 21589, Saudi Arabia
2Chemistry Department, Faculty of Science, Mansoura University, 35516 - Mansoura, Egypt
Correspondence to: Magdy Y. Abdelaal , Chemistry Department, Faculty of Science, King Abdulaziz University, PO Box 80203, Jeddah 21589, Saudi Arabia.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Polyacrylic acid was converted into modified polymers with the targeted functional groups through the reaction with different organic compounds such as some aromatic amines and acids. The obtained modified polymeric products have been characterized through FT-IR spectroscopic and thermal analyses. Modified acrylic polymers were tested for their ability to be used for heavy metal ion recovery from their aqueous solutions. They also were tested as polymeric antioxidants for PVC as a standard polymer by using the discoloration extent of PVC as a function of thermal degradation of PVC due to the thermal treatment. The obtained results were discussed and justified.
Keywords: Modification, Polyacrylic Acid, Metal Ion Recovery, Thermal Stability, Aromatic Amines
Article Outline
1. Introduction
- Polyacrylic acid (PAA) belongs to the class of commercial polymers produced on a large scale and widely used in various industries, agriculture, and medicine[1]. PAA, its salts and PAA-based polymeric materials are used as emulsifiers and thickening agents for aqueous solutions and dispersions of both natural and synthetic latexes, smoothing agents for synthetic fibers, sorbents and ion exchangers, aqueous quenching media, flocculants, plastics, etc.[2-5]. Copolymers of acrylamide and divinylbenzene are used as ion exchange resins as well as copolymers of acrylic acids with acrylamide and polyvinyl alcohol are known for their activity toward metal ion uptake to be applied in the metal ion preconcentration or removal[6]. Polymers and copolymers of acrylic acid are known as efficient flocculants used, in particular, for increasing the strength of special paper grades[7].Hydrophilic properties of PAA explain its wider use in agriculture for presuming treatment of seeds, etc. In recent years, there has been a growing interest in PAA as a basis for obtaining various physiologically active polymeric substances capable of separating from the carrier molecule at definite site of the living organism and producing a targeted physiological action[8].Both PAA and the related compounds are more widely used as polymeric carriers for proteins, enzymes, drugs and other biologically active substances. In the latter case, PAA can be used for solving two mutually related problems of the chemistry and technology of biopolymer for medical applications[9]. The two problems are creation of synthetic polymeric materials for short-time or prolonged contact with blood and other tissues in the living organism and development of the synthesis methods, establishing the mechanisms of physiological action of the macromolecules of pharmaceutical preparations[10].Microwave initiated grafting of polyacrylic acid onto carboxymethylcellulose (CMC) to synthesize CMC-g-PAA which has showed higher flocculation efficiency than CMC itself reflecting the role of polyacrylic acid in such application. The high flocculation efficiency of polyacrylic acid grafted CMC makes it a good candidate as flocculants for river water clarification[11].Synthesis of PAA and its properties have been extensively studied and repeatedly reviewed. It will be only mentioned briefly some points of importance. PAA is obtained by polymerization of acrylic acid (AA) in the presence of photo-initiators or under the action γ-radiation[12]. In the solid state, AA converts into polymer on exposure to UV radiation. Hydrogen peroxide, alkali metal or ammonium persulfates, and cumene hudrogen peroxide initiate the polymerization of AA in aqueous solution. Only non-ionized AA molecules enter into the polymerization reaction and the minimum reaction rate is observed.Aqueous media are preferred for the polymerization of AA whose concentration should not exceed 25% due to the exothermic nature of the reaction which is otherwise difficult to control. Polymerization of AA in non-aqueous solvents is initiated by organic peroxides, AIBN and some redox and other systems.The most active polymers on the basis of esters, anhydrides, amides, and related PAA derivatives composed of the –CH2-CH(C(=O)X)- units, where X is OR, OCOR, NH2, NR2, etc., can be synthesized using three pathways. First is grafting of the reactive physiologically active monomers onto PAA matrix with the formation of valence bonds. An example is the synthesis of some sulfanilamide preparations, hormones, and some other biologically active compounds with reactive OH or NH2 groups[13]. The second one is the condensation of polyacrylyl chloride,polyacrylamide, polyalkylacrylate, or polyacrylic acid salt with physiologically active substance containing thecorresponding functional groups. The last one is thehomo-polymerization of physiologically active acrylic acid substituent of the formula CH2=CH(C(=O)Y)- where Y is a pharmacophore group bound through oxygen or nitrogen atom.The first investigations in the synthesis of PAA derivatives containing reactive functional groups were reported by Kern and Schulz[12,14]. By interaction of polymethacrylate with hydrazine they obtained a polyacryloylhydrazide which was converted by Curtius reaction into polyacryloylazide. This was followed by condensation with various carbonyl compounds yielding polyacryloylhydrazones. A reaction of polymethylacrylate with hydroxylamine led to polyacryloylhydroxamic acid. Interaction of the latter acid with the salts of various metal ions led to a series of the corresponding metal containing derivatives. There are many trials to prepare modified polyacrylic acids through the synthesis of modified monomers followed by their polymerization[15]. Although modified polymers with higher purity would be obtained, the chemical modification of the monomers is not simple and need many precautions beside it is not suitable for the recycling of polyacrylic acid wastes. The current work is aiming to preparation of different polyacrylic acidderivatives through simple methodologies followed bycharacterization and utilization of them as polymeric anti-oxidant and as a tool for recovery of heavy metals from their aqueous solutions.
2. Experimental Part
2.1. Materials and Techniques
- All chemicals were obtained from Aldrich and used as received without further purification unless otherwise mentioned. PVC (M.Wt.=100.000) was also obtained from Aldrich.FT-IR Spectroscopic analysis was carried out using a Perkin-Elmer 1430 spectrophotometer in the range of 4000-400 cm-1. UV spectra were recorded on a double beam spectrophotometer to measure the extent of discoloration of the degraded PVC samples as a function of degradation time. ICP/AES in the department was used to determine the concentration of the heavy metal ions under investigation. Differential thermal analysis (DTA) was carried out with a Shimadzu thermo-analyzer (D-50), Japan. Samples were heated at 10 C/min in a stream of dry nitrogen using sintered alumina (α-Al2O3) as thermally inert reference material.
2.2. Synthesis of Polyacrylic Acid
- A solution of 0.2 mole of dibenzoylperoxide in THF was added to 2 mole of acrylic acid solution in THF while stirring. The mixture was purged with nitrogen for 30 min at 70C and was continued for 30 min further at the same conditions before cooling to the room temperature. Polyacrylic acid was precipitated by pouring into 400 ml of anhydrous diethyl ether. The obtained product was filtered off and washed successively with diethyl ether and methanol then dried overnight under vacuum at 40 °C.
2.3. Synthesis of Polyacryly Chloride
- About 1.4 mole of thionyl chloride was added dropwisely to 1 mole of polyacrylic acid in a round bottomed flask. The reaction mixture was stirred for 1 h at 60°C and left to cool to the room temperature. The unreacted thionyl chloride was collected by filtration. The obtained product was washed with methylene chloride and dried overnight in vacuum at 40 °C.
2.4. Synthesis of Poly-2-acryloxybenzoic Acid
- A solution of 0.25 mole salicylic acid and 0.75 mole of triethylamine in 300 ml acetonitrite was added dropwise to 0.3 mole of polyacrylyl chloride pre-soaked in 100 ml acetonitrite for 6 h. After complete addition, the reaction mixture was stirred further for 6 h at 25C. The reaction mixture was filtered off and washed with methanol, 1M HCl, distilled water to remove the formed triethylamine hydrochloride and finally with diethyl ether. The obtained polyacryloxybenzoic acid was dried overnight under vacuum at 40 °C.
2.5. Synthesis of Poly-N-acroylaniline
- A cold solution of 0.07 mole of aniline in 100 methylene chloride was added portion-wise with stirring to 0.03 mole of polyacrylyl chloride soaked in 50 ml methylene chloride. After the complete addition stirring was continued for 30 min further and the product was filtered off. The excess of aniline was removed by washing the precipitate with methylene chloride, acetone and methanol respectively and the obtained product was dried overnight under vacuum at 40 C. In the same way, poly-N-acrylyl-8-aminoquinolione and poly-N-acrylylnaphtylamine were prepared.
2.6. Synthesis of Poly-4-acryloxybenzalthiobarbituric Acid
- 0.2 mole of polyacrylyl chloride was dissolved in THF and added drop-wise to a cold solution of 0.2 mole of 4-hydroxybenzalthiobarbituric acid and 20ml oftriethylamine in 100 ml THF and stirred further for 24 h at room temperature. The reaction mixture was poured portion-wise into dilute solution of HCl. The formed precipitate was washed thoroughly with subsequent amounts of dilute HCl solution, methanol, acetone and diethyl ether. The product was then finally dried overnight under vacuum at 40 C.
2.7. Effect of Modified Polyacrylic Acid on Thermal Stability of PVC
- A solution of 10 g PVC was dissolved in 150 ml THF was mixed with 0.2 g of modified polyacrylic acid dissolved in THF with stirring followed by sonication of the mixture to remove all the trapped gases during mixing. After that, the mixture was casted on a polypropylene sheet mounted on a horizontal glass plate. After evaporation of the solvents at room temperature, the obtained film was dried overnight under vacuum at room temperature. Modified polyacrylic acids used were polyacrylyloxybenzoic acid and polyacrylyloxybenzalthiobarbituric acid. The obtained film was then cut into strips and thermally treated at 100 C for different intervals.The discoloration extent of PVC blends attributed to dehydrohalogenation of PVC[16] was used as a function of thermal degradation of PVC and is related to the absorbance at λ =400nm for the blended films in comparison with a blank sample of PVC under comparable conditions. The absorbance was plotted against time of thermal treatment of PVC blends and calculated as an average of 3 replicates. Data of the investigation is summarized in Table 1.
|
2.8. Metal Ion Recovery of Poly-2-acrylyloxybenzoic Acid
- 0.1 g portions of polyacrylyloxybenzoic acid were added separately to 100 ml solution of the studied heavy metal ions in 100 mg/l concentration after adjusting its pH at ~4.5. These solutions were shaken for 1 h. The polymer was filtered off and washed with double distilled water. The sorbed metal ions were eluted with 5 ml of 2M HCl and the metal ion uptake of the polymer (in mg/g) was calculated after determination of the eluted metal ions by ICP/AES technique.
3. Results and Discussion
3.1. Characterization of Poly-2-acrylyloxybenzoic Acid
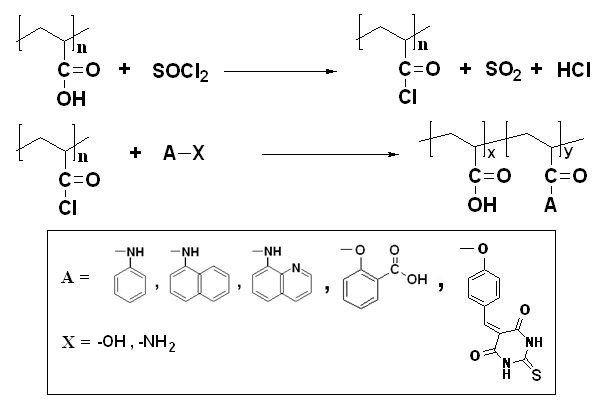
- Chemical modification of polyacrylic acid with benzoic acid was performed according to Scheme 1.FT-IR spectroscopic analysis in Figure 1 showed characteristic absorptions related to polyacrylic acid in addition to the absorption corresponding to the aromatic moiety at 1615 cm-1 and 1455 cm-1 beside the absorption of free carboxylic and ester C=O groups at 1690 cm-1 and at 1740 cm-1, respectively and OH stretching at 3030 cm-1.
 | Scheme 1. Chemical modification of polyacrylic acid |
 | Figure 1. IR curve of poly-2-acryloxybenzoic acid |
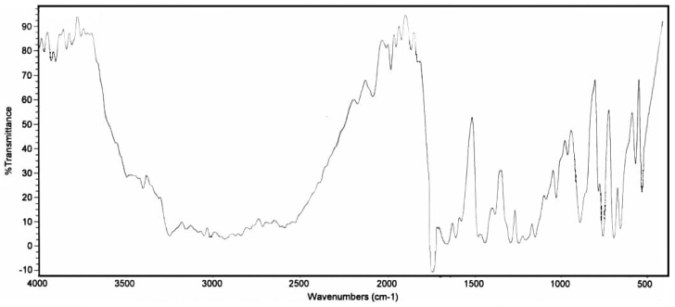
 | Figure 2. TGA of poly-2-acryloxybenzoic acid |
3.2. Characterization of Poly-N-acrylylamines
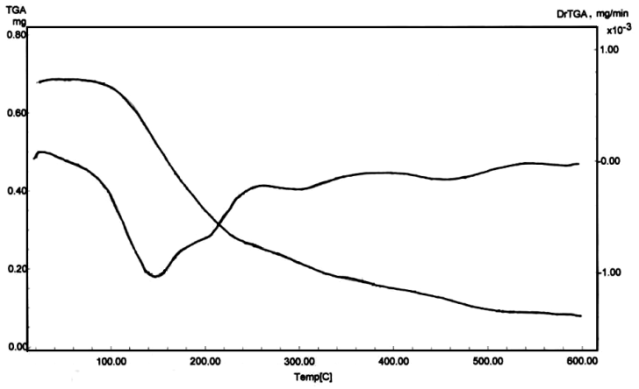
- Chemical modification of polyacrylyl chloride with different amines such as aniline, α-naphthylamine and 8-aminoquinoline was carried out according Scheme 1. FT-IR spectrum of poly-N-acrylylaniline in Figure 3a shows absorption band for COCl at 1660 cm-1 is absent, while the absorption bands of N-H stretching and amidic C=O groups appear at 3270 cm-1 and at 1610 cm-1, respectively indicating the successful modification of polyacrylyl chloride with aniline into polumer-supported amines. The same findings hold true for poly-N-acrylyl-α-naphthylamine. There are also absorption bands at 1626 cm-1 related to C=C (olefinic) and at 1570 cm-1 related to C=N (aromatic) for poly-N-acrylyl-8-aminoquinoline shown in Figure 3b.
 | Figure 3. IR spectroscopic analysis of (a) poly-N-acrylylaniline and (b) poly-N-acryloyl-8-aminoqionoline |
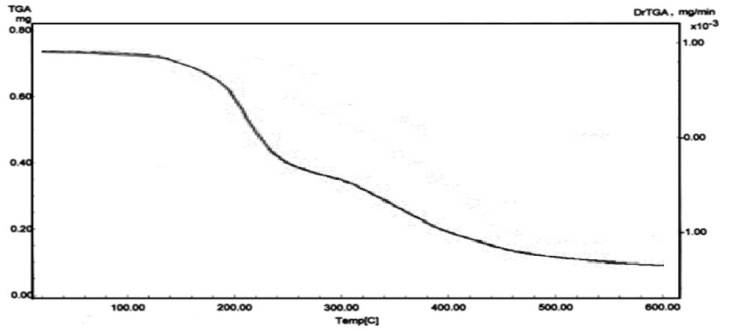 | Figure 4. TGA polyacrylic acid |
3.3. Characterization of Poly-4-acrylyloxybenzalthiobarbituric Acid
- Polyacrylyl chloride was reactedwith4-hydroxybenzal-thiobarbituric acid to form polyacrylyloxybenzalthiobarbituric acid shown in Scheme 1. FT-IR spectroscopic analysis in Figure 5 showed absorption bands at 1170 cm-1 related to C=S bonds and at 1680 cm-1 and 1726 cm-1 related to C=O (ketone) and C=O (ester) groups, respectively.
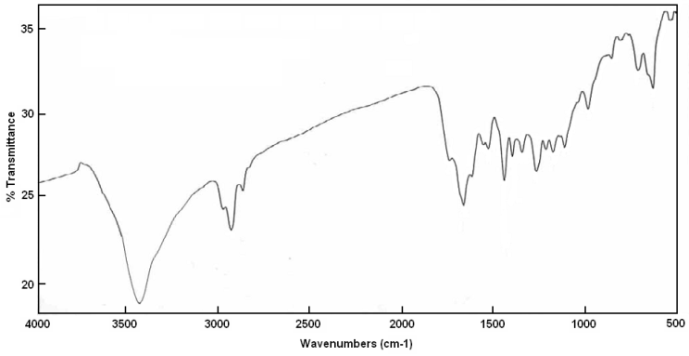 | Figure 5. IR curve of poly-4-acryloxybenzalthiobabituric acids |
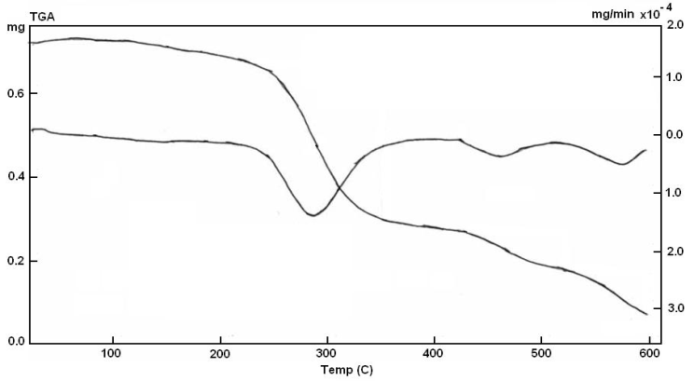 | Figure 6. TGA of poly-4-acrylyloxybenzalthiobabituric acids |
3.4. Stabilizing Effect of Modified Polyacrylic Acid on PVC
- Poly-4-acrylyloxybenzalthiobarbituric acid andpolyacrylyloxybenzoic acid and were used as thermal stabilizers against autocatalytic dehydrochlorination reaction of PVC in presence of modified acrylic polymers. This reaction causes degradation of PVC which leads to a severe discoloration and lack of mechanical properties of PVC[16]. Thiobarbituric acid-supported polymer possesses a variety of functional groups able to interfere with the degradation process and also to absorb HCl produced by degradation of PVC. This results in reduction or delay of the harmful effect of acidic degradation products[17].This can be proved by the stabilizing efficiency of modified polyacrylic acids on the treated PVC reflected by reducing the extent of discoloration of induced by thermal treatment of PVC in comparison with blank PVC. This can be attributed to the dehydrohalogenation of PVC under thermal treatment conditions[16,18,19]. Figure 7 shows a lower extent of discoloration of the treated PVC samples relative to blank sample. These results confirm a mechanism involving an interaction of the thiobarbituric acid moiety (OBT) from the polymer with the chlorine atoms emitted from the polymer and thus blocks the odd electron sites on PVC chains.This action suppresses the possibility for conjugation rather than with HCl evolved. Presence of thiobarbituric acid with a spacer group of oxybenzal moiety helps for higher mobility of such side chains to catch emitted HCl relative to the directly attached thiobarbituric acid moiety to polymeric backbone.
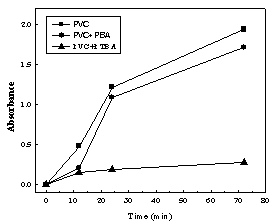 | Figure 7. Time dependence of absorbance a function of discoloration extent of PVC heated at 100 °C in presence of modified polyacrylic acid as thermal stabilizer |
3.5. Application of Modified Polyacrylic Acid in of Metal Ion Recovery
- Modified polyacrylic acid with benzoic acid functionality was selected to be investigated for the recovery of metal ions from their aqueous solutions acting as ion exchanger or chelating agent. The sorption capacity ofpolyacrylyloxybenzoic acid in mg/g for Cd(II), Cu(II), Fe(III) and Co(II) was 35.4, 38.5, 31, 40 mg/g polymer, respectively, which is obviously higher than that for the polyacrylic acid without modification which showed a capacity of 20 mg/g polymer. The sorption process was adjusted at pH = 4.5 for all the investigated metal ions under stirring for 30 min and using 2M HCl solution for elution of the metal ions out of the polymer matrix. From this investigation it is shown the different abilities of the modified polyacrylic acid to recover different metal ions from their aqueous solutions.
4. Conclusions and Recommendations
- It can be concluded that the modified polyacrylic acid can be promising in many applications.Poly-2-acrylyloxybenzoic acid showed thermal stability up to 250 C. Poly-N-acrylylaniline showed also thermal stability up to 235 C while it was up to 265 C for poly-N-acrylyl-α-naphthylamine. Beside thermal stability up to 235 C, polyacrylyloxybenzalthiobarbituric acid showed also ability to be used as thermal stabilizer for PVC.Polyacrylyloxybenzoic acid showed different abilities to recover different metal ions from their aqueous solutions which can be applied in many application directions such as metal ion recovery from their aqueous solutions.
ACKNOWLEDGEMENTS
- This project was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under grant no. 418/130/431. The authors, therefore, acknowledge with thank DSR technical and financial support.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML